Korean J Physiol Pharmacol.
2021 Mar;25(2):131-137. 10.4196/kjpp.2021.25.2.131.
Mangiferin ameliorates cardiac fibrosis in D-galactose-induced aging rats by inhibiting TGF-β/p38/MK2 signaling pathway
- Affiliations
-
- 1Department of Pharmacy, Wuhan Fourth Hospital, Puai Hospital, Tongji Medical College, Huazhong University of Science and Technology, Xian 710054, Shanxi, China
- 2Dong Xi Hu Municipal Healthcare Security Administration, Xian 710054, Shanxi, China
- 3Department of Orthopedics, Wuhan Fourth Hospital, Puai Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430033, Hubei, Xian 710054, Shanxi, China
- 4Department of Traditional Chinese Medicine, 986 Hospital of Air Force, Xian 710054, Shanxi, China
- KMID: 2512956
- DOI: http://doi.org/10.4196/kjpp.2021.25.2.131
Abstract
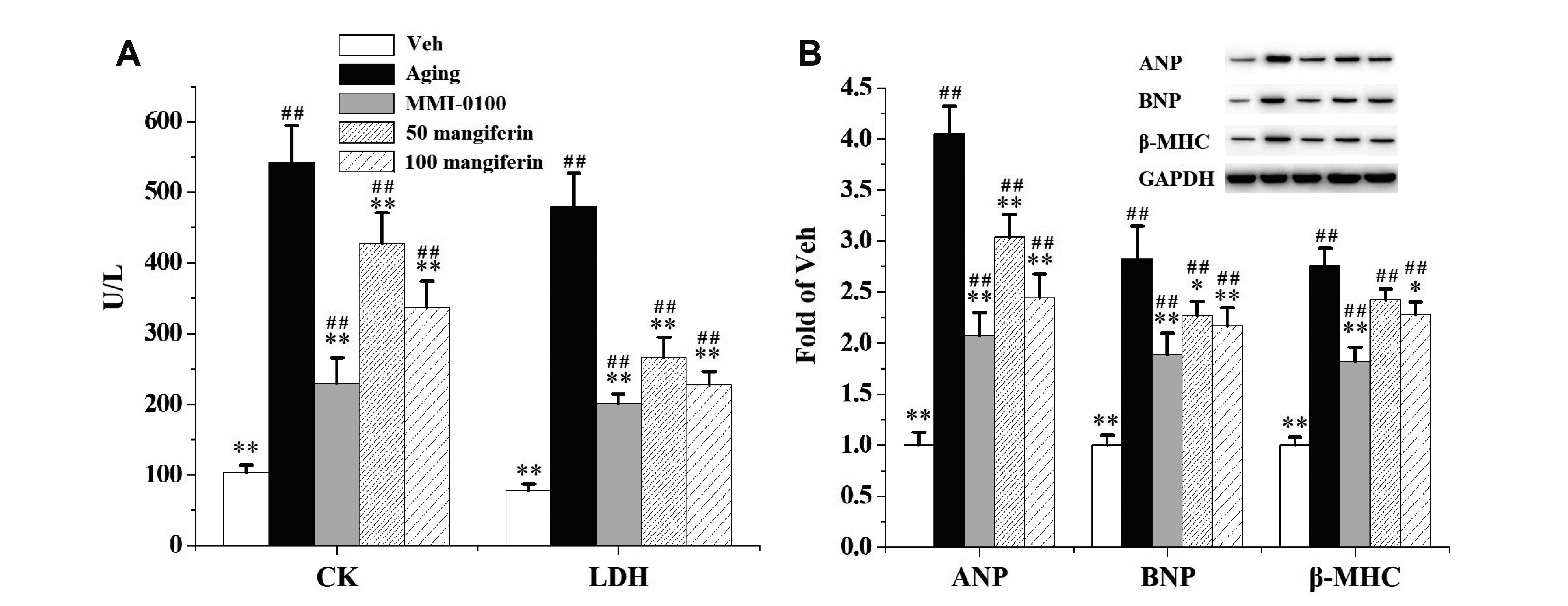
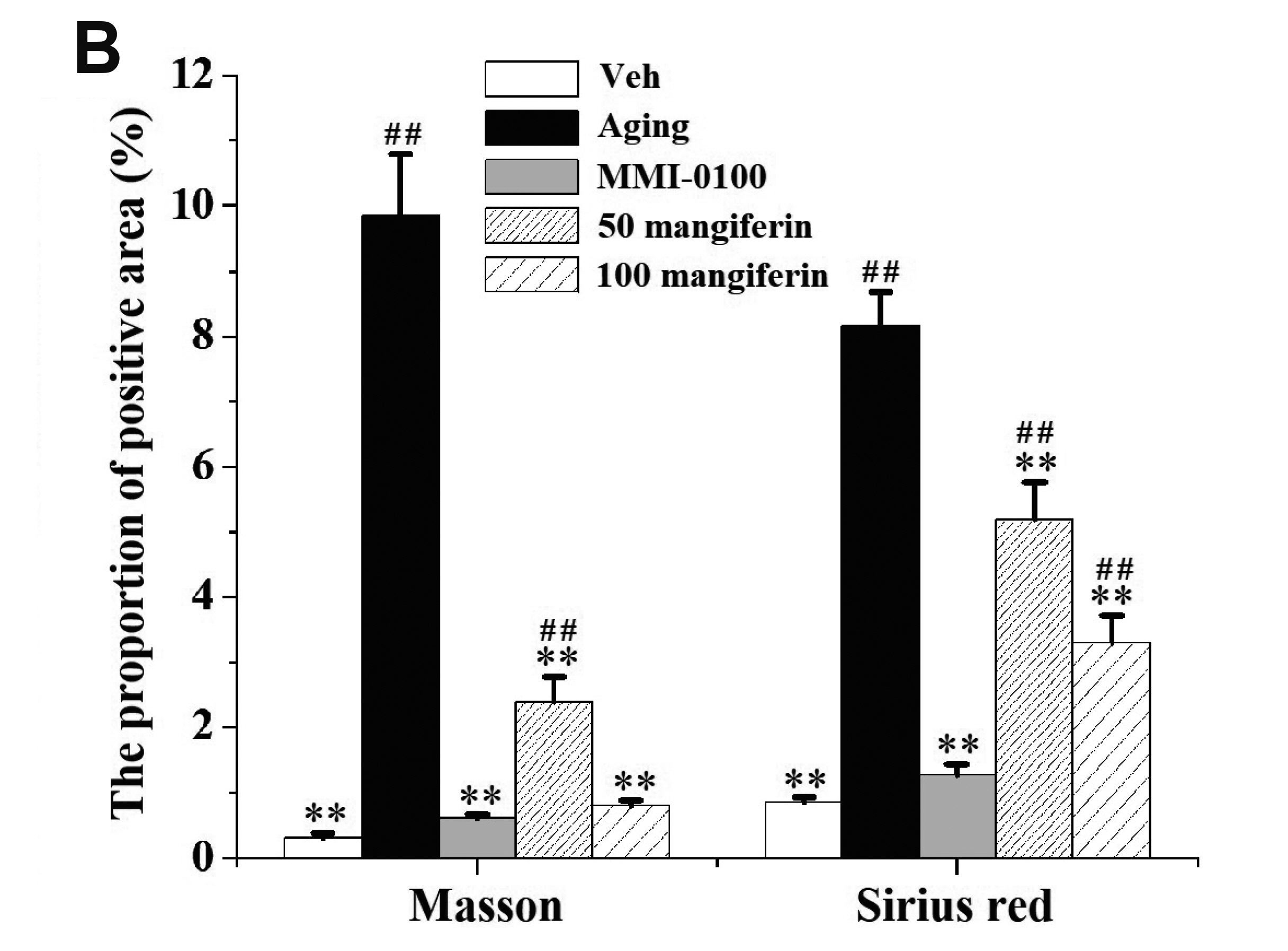
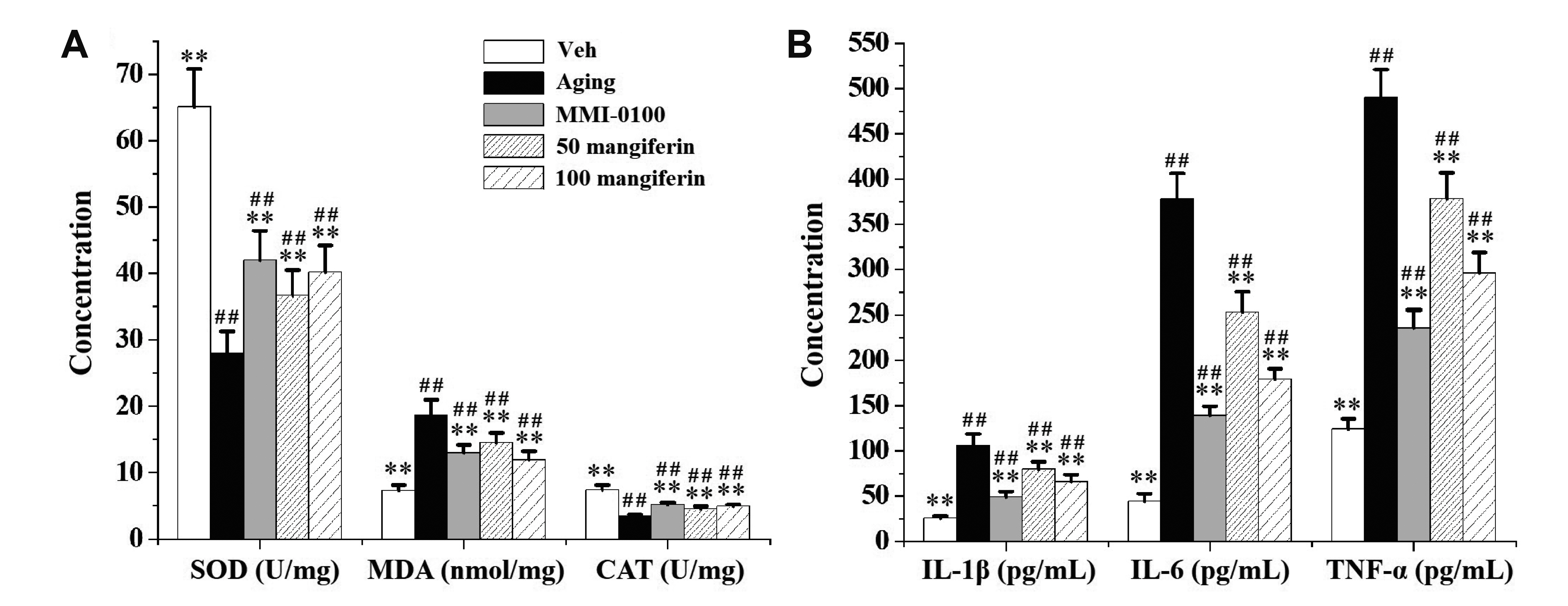
- Aging is the process spontaneously occurred in living organisms. Cardiac fibrosis is a pathophysiological process of cardiac aging. Mangiferin is a wellknown C-glucoside xanthone in mango leaves with lots of beneficial properties. In this study, rat model of cardiac fibrosis was induced by injected with 150 mg/kg/d Dgalactose for 8 weeks. The age-related cardiac decline was estimated by detecting the relative weight of heart, the serum levels of cardiac injury indicators and the expression of hypertrophic biomakers. Cardiac oxidative stress and local inflammation were measured by detecting the levels of malondialdehyde, enzymatic antioxidant status and proinflammatory cytokines. Cardiac fibrosis was evaluated by observing collagen deposition via masson and sirius red staining, as well as by examining the expression of extracellular matrix proteins via Western blot analysis. The cardiac activity of profibrotic TGF-β1/p38/MK2 signaling pathway was assessed by measuring the expression of TGF-β1 and the phosphorylation levels of p38 and MK2. It was observed that mangiferin ameliorated D-galactose-induced cardiac aging, attenuated cardiac oxidative stress, inflammation and fibrosis, as well as inhibited the activation of TGF-β1/p38/MK2 signaling pathway. These results showed that mangiferin could ameliorate cardiac fibrosis in D-galactose-induced aging rats possibly via inhibiting TGF-β/p38/MK2 signaling pathway.
Keyword
Figure
Reference
-
1. Bo-Htay C, Palee S, Apaijai N, Chattipakorn SC, Chattipakorn N. 2018; Effects of D-galactose-induced ageing on the heart and its potential interventions. J Cell Mol Med. 22:1392–1410. DOI: 10.1111/jcmm.13472. PMID: 29363871. PMCID: PMC5824366.
Article2. Zhao X, Yi R, Zhou X, Mu J, Long X, Pan Y, Song JL, Park KY. 2019; Preventive effect of Lactobacillus plantarum KSFY02 isolated from naturally fermented yogurt from Xinjiang, China, on D-galactose-induced oxidative aging in mice. J Dairy Sci. 102:5899–5912. DOI: 10.3168/jds.2018-16033. PMID: 31103296.
Article3. Althurwi HN, Abdel-Kader MS, Alharthy KM, Salkini MA, Albaqami FF. 2020; Cymbopogon proximus essential oil protects rats against isoproterenol-induced cardiac hypertrophy and fibrosis. Molecules. 25:1786. DOI: 10.3390/molecules25081786. PMID: 32295062. PMCID: PMC7221672.
Article4. Deng M, Yang S, Ji Y, Lu Y, Qiu M, Sheng Y, Sun W, Kong X. 2020; Overexpression of peptidase inhibitor 16 attenuates angiotensin II-induced cardiac fibrosis via regulating HDAC1 of cardiac fibroblasts. J Cell Mol Med. 24:5249–5259. DOI: 10.1111/jcmm.15178. PMID: 32227584. PMCID: PMC7205788.
Article5. Chen WK, Tsai YL, Shibu MA, Shen CY, Chang-Lee SN, Chen RJ, Yao CH, Ban B, Kuo WW, Huang CY. 2018; Exercise training augments Sirt1-signaling and attenuates cardiac inflammation in D-galactose induced-aging rats. Aging (Albany NY). 10:4166–4174. DOI: 10.18632/aging.101714. PMID: 30582744. PMCID: PMC6326662.
Article6. Dehghani A, Hafizibarjin Z, Najjari R, Kaseb F, Safari F. 2019; Resveratrol and 1,25-dihydroxyvitamin D co-administration protects the heart against D-galactose-induced aging in rats: evaluation of serum and cardiac levels of klotho. Aging Clin Exp Res. 31:1195–1205. DOI: 10.1007/s40520-018-1075-x. PMID: 30484255.
Article7. Chang YM, Chang HH, Lin HJ, Tsai CC, Tsai CT, Chang HN, Lin SL, PadmaViswanadha V, Chen RJ, Huang CY. 2017; Inhibition of cardiac hypertrophy effects in D-galactose-induced senescent hearts by Alpinate Oxyphyllae Fructus treatment. Evid Based Complement Alternat Med. 2017:2624384. DOI: 10.1155/2017/2624384. PMID: 28479925. PMCID: PMC5396449.8. Murtha LA, Morten M, Schuliga MJ, Mabotuwana NS, Hardy SA, Waters DW, Burgess JK, Ngo DT, Sverdlov AL, Knight DA, Boyle AJ. 2019; The role of pathological aging in cardiac and pulmonary fibrosis. Aging Dis. 10:419–428. DOI: 10.14336/AD.2018.0601. PMID: 31011486. PMCID: PMC6457057.
Article9. Zhu B, Zhang L, Liang C, Liu B, Pan X, Wang Y, Zhang Y, Zhang Y, Xie W, Yan B, Liu F, Yip HK, Yu XY, Li Y. 2019; Stem cell-derived exosomes prevent aging-induced cardiac dysfunction through a novel exosome/lncRNA MALAT1/NF-κB/TNF-α signaling pathway. Oxid Med Cell Longev. 2019:9739258. DOI: 10.1155/2019/9739258. PMID: 31089420. PMCID: PMC6476062.
Article10. Guan W, Liu Y, Liu Y, Wang Q, Ye HL, Cheng YG, Kuang HX, Jiang XC, Yang BY. 2019; Proteomics research on the protective effect of mangiferin on H9C2 cell injury induced by H2O2. Molecules. 24:1911. DOI: 10.3390/molecules24101911. PMID: 31109015. PMCID: PMC6572523.11. Suchal K, Malik S, Gamad N, Malhotra RK, Goyal SN, Ojha S, Kumari S, Bhatia J, Arya DS. 2016; Mangiferin protect myocardial insults through modulation of MAPK/TGF-β pathways. Eur J Pharmacol. 776:34–43. DOI: 10.1016/j.ejphar.2016.02.055. PMID: 26921754.
Article12. Jiang T, Han F, Gao G, Liu M. 2020; Mangiferin exert cardioprotective and anti-apoptotic effects in heart failure induced rats. Life Sci. 249:117476. DOI: 10.1016/j.lfs.2020.117476. PMID: 32119962.
Article13. Song J, Meng Y, Wang M, Li L, Liu Z, Zheng K, Wu L, Liu B, Hou F, Li A. 2020; Mangiferin activates Nrf2 to attenuate cardiac fibrosis via redistributing glutaminolysis-derived glutamate. Pharmacol Res. 157:104845. DOI: 10.1016/j.phrs.2020.104845. PMID: 32353588.
Article14. Chang YM, Tamilselvi S, Lin HJ, Tsai CC, Lin YM, Day CH, Viswanadha VP, Chang HN, Kuo WW, Huang CY. 2019; Alpinia oxyphylla Miq extract ameliorates cardiac fibrosis associated with D-galactose induced aging in rats. Environ Toxicol. 34:172–178. DOI: 10.1002/tox.22671. PMID: 30367734.
Article15. Meng Q, Bhandary B, Osinska H, James J, Xu N, Shay-Winkler K, Gulick J, Willis MS, Lander C, Robbins J. 2017; MMI-0100 inhibits cardiac fibrosis in a mouse model overexpressing cardiac myosin binding protein C. J Am Heart Assoc. 6:e006590. DOI: 10.1161/JAHA.117.006590. PMID: 28871043. PMCID: PMC5634300.
Article16. Li Q, Jiang W, Wan Z, Ni Y, Lei L, Wei J. 2020; Polyphyllin I attenuates pressure over-load induced cardiac hypertrophy via inhibition of Wnt/β-catenin signaling pathway. Life Sci. 252:117624. DOI: 10.1016/j.lfs.2020.117624. PMID: 32259602.
Article17. Chen Y, Ge Z, Huang S, Zhou L, Zhai C, Chen Y, Hu Q, Cao W, Weng Y, Li Y. 2020; Delphinidin attenuates pathological cardiac hypertrophy via the AMPK/NOX/MAPK signaling pathway. Aging (Albany NY). 12:5362–5383. DOI: 10.18632/aging.102956. PMID: 32209725. PMCID: PMC7138591.
Article18. Jia L, Sun P, Gao H, Shen J, Gao Y, Meng C, Fu S, Yao H, Zhang G. 2019; Mangiferin attenuates bleomycin-induced pulmonary fibrosis in mice through inhibiting TLR4/p65 and TGF-β1/Smad2/3 pathway. J Pharm Pharmacol. 71:1017–1028. DOI: 10.1111/jphp.13077. PMID: 30847938.
Article19. Song Y, Liu W, Tang K, Zang J, Li D, Gao H. 2020; Mangiferin alleviates renal interstitial fibrosis in streptozotocin-induced diabetic mice through regulating the PTEN/PI3K/Akt signaling pathway. J Diabetes Res. 2020:9481720. DOI: 10.1155/2020/9481720. PMID: 32076626. PMCID: PMC7016412.
Article20. Li X, Yan Z, Carlström M, Tian J, Zhang X, Zhang W, Wu S, Ye F. 2020; Mangiferin ameliorates hyperuricemic nephropathy which is associated with downregulation of AQP2 and increased urinary uric acid excretion. Front Pharmacol. 11:49. DOI: 10.3389/fphar.2020.00049. PMID: 32116724. PMCID: PMC7020245.
Article21. Li C, Yan Q, Tang S, Xiao W, Tan Z. 2018; L-Theanine protects H9C2 cells from hydrogen peroxide-induced apoptosis by enhancing antioxidant capability. Med Sci Monit. 24:2109–2118. DOI: 10.12659/MSM.907660. PMID: 29629712. PMCID: PMC5907829.
Article22. Al-Awaida W, Akash M, Aburubaiha Z, Talib WH, Shehadeh H. 2014; Chinese green tea consumption reduces oxidative stress, inflammation and tissues damage in smoke exposed rats. Iran J Basic Med Sci. 17:740–746. PMID: 25729541. PMCID: PMC4340980.23. Liu J, Ai Y, Niu X, Shang F, Li Z, Liu H, Li W, Ma W, Chen R, Wei T, Li X, Li X. 2020; Taurine protects against cardiac dysfunction induced by pressure overload through SIRT1-p53 activation. Chem Biol Interact. 317:108972. DOI: 10.1016/j.cbi.2020.108972. PMID: 32017914.
Article24. Leong XY, Thanikachalam PV, Pandey M, Ramamurthy S. 2016; A systematic review of the protective role of swertiamarin in cardiac and metabolic diseases. Biomed Pharmacother. 84:1051–1060. DOI: 10.1016/j.biopha.2016.10.044. PMID: 27780133.
Article25. Palomer X, Román-Azcona MS, Pizarro-Delgado J, Planavila A, Villarroya F, Valenzuela-Alcaraz B, Crispi F, Sepúlveda-Martínez Á, Miguel-Escalada I, Ferrer J, Nistal JF, García R, Davidson MM, Barroso E, Vázquez-Carrera M. 2020; SIRT3-mediated inhibition of FOS through histone H3 deacetylation prevents cardiac fibrosis and inflammation. Signal Transduct Target Ther. 5:14. DOI: 10.1038/s41392-020-0114-1. PMID: 32296036. PMCID: PMC7046732.
Article26. El-Baz FK, Hussein RA, Saleh DO, Abdel Jaleel GAR. 2019; Zeaxanthin isolated from Dunaliella salina microalgae ameliorates age associated cardiac dysfunction in rats through stimulation of retinoid receptors. Mar Drugs. 17:290. DOI: 10.3390/md17050290. PMID: 31091726. PMCID: PMC6562725.27. Weiskirchen R, Weiskirchen S, Tacke F. 2019; Organ and tissue fibrosis: molecular signals, cellular mechanisms and translational implications. Mol Aspects Med. 65:2–15. DOI: 10.1016/j.mam.2018.06.003. PMID: 29958900.
Article28. Zhou B, Yu JW. 2017; A novel identified circular RNA, circRNA_010567, promotes myocardial fibrosis via suppressing miR-141 by targeting TGF-β1. Biochem Biophys Res Commun. 487:769–775. DOI: 10.1016/j.bbrc.2017.04.044. PMID: 28412345.
Article29. Yang L, Wang B, Zhou Q, Wang Y, Liu X, Liu Z, Zhan Z. 2018; MicroRNA-21 prevents excessive inflammation and cardiac dysfunction after myocardial infarction through targeting KBTBD7. Cell Death Dis. 9:769. DOI: 10.1038/s41419-018-0805-5. PMID: 29991775. PMCID: PMC6039462.
Article30. Tan S, Lu Y, Xu M, Huang X, Liu H, Jiang J, Wu B. 2019; β-Arrestin1 enhances liver fibrosis through autophagy-mediated Snail signaling. FASEB J. 33:2000–2016. DOI: 10.1096/fj.201800828RR. PMID: 30216111.
Article31. Tanaka T, Iino M, Goto K. 2018; Sec6 enhances cell migration and suppresses apoptosis by elevating the phosphorylation of p38 MAPK, MK2, and HSP27. Cell Signal. 49:1–16. DOI: 10.1016/j.cellsig.2018.04.009. PMID: 29729335.
Article32. Xu L, Yates CC, Lockyer P, Xie L, Bevilacqua A, He J, Lander C, Patterson C, Willis M. 2014; MMI-0100 inhibits cardiac fibrosis in myocardial infarction by direct actions on cardiomyocytes and fibroblasts via MK2 inhibition. J Mol Cell Cardiol. 77:86–101. DOI: 10.1016/j.yjmcc.2014.09.011. PMID: 25257914. PMCID: PMC4312211.
Article33. Singh RK, Najmi AK. 2019; Novel therapeutic potential of mitogen-activated protein kinase activated protein kinase 2 (MK2) in chronic airway inflammatory disorders. Curr Drug Targets. 20:367–379. DOI: 10.2174/1389450119666180816121323. PMID: 30112991.
Article34. Wang Z, Liang XY, Chang X, Nie YY, Guo C, Jiang JH, Chang M. 2019; MMI-0100 ameliorates dextran sulfate sodium-induced colitis in mice through targeting MK2 pathway. Molecules. 24:2832. DOI: 10.3390/molecules24152832. PMID: 31382637. PMCID: PMC6696270.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mangiferin ameliorates cardiac fibrosis in D-galactose-induced aging rats by inhibiting TGF-β/p38/MK2 signaling pathway
- The flavonoid fisetin ameliorates renal fibrosis by inhibiting SMAD3 phosphorylation, oxidative damage, and inflammation in ureteral obstructed kidney in mice
- Hydrogen sulfide alleviates hypothyroidism-induced myocardial fibrosis in rats through stimulating autophagy and inhibiting TGF-β1/Smad2 pathway
- Hydrogen sulfide ameliorates abdominal aorta coarctationinduced myocardial fibrosis by inhibiting pyroptosis through regulating eukaryotic translation initiation factor 2αα phosphorylation and activating PI3K/AKT1 pathway
- TGF-beta-activated kinase-1: New insights into the mechanism of TGF-beta signaling and kidney disease