Korean J Physiol Pharmacol.
2023 Jul;27(4):345-356. 10.4196/kjpp.2023.27.4.345.
Hydrogen sulfide ameliorates abdominal aorta coarctationinduced myocardial fibrosis by inhibiting pyroptosis through regulating eukaryotic translation initiation factor 2αα phosphorylation and activating PI3K/AKT1 pathway
- Affiliations
-
- 1Department of Cardiology, Zhuzhou Central Hospital, Zhuzhou 412000, China
- 2Department of Cardiology, The First Affiliated Hospital of University of South China, Hengyang 421001, China
- KMID: 2544136
- DOI: http://doi.org/10.4196/kjpp.2023.27.4.345
Abstract
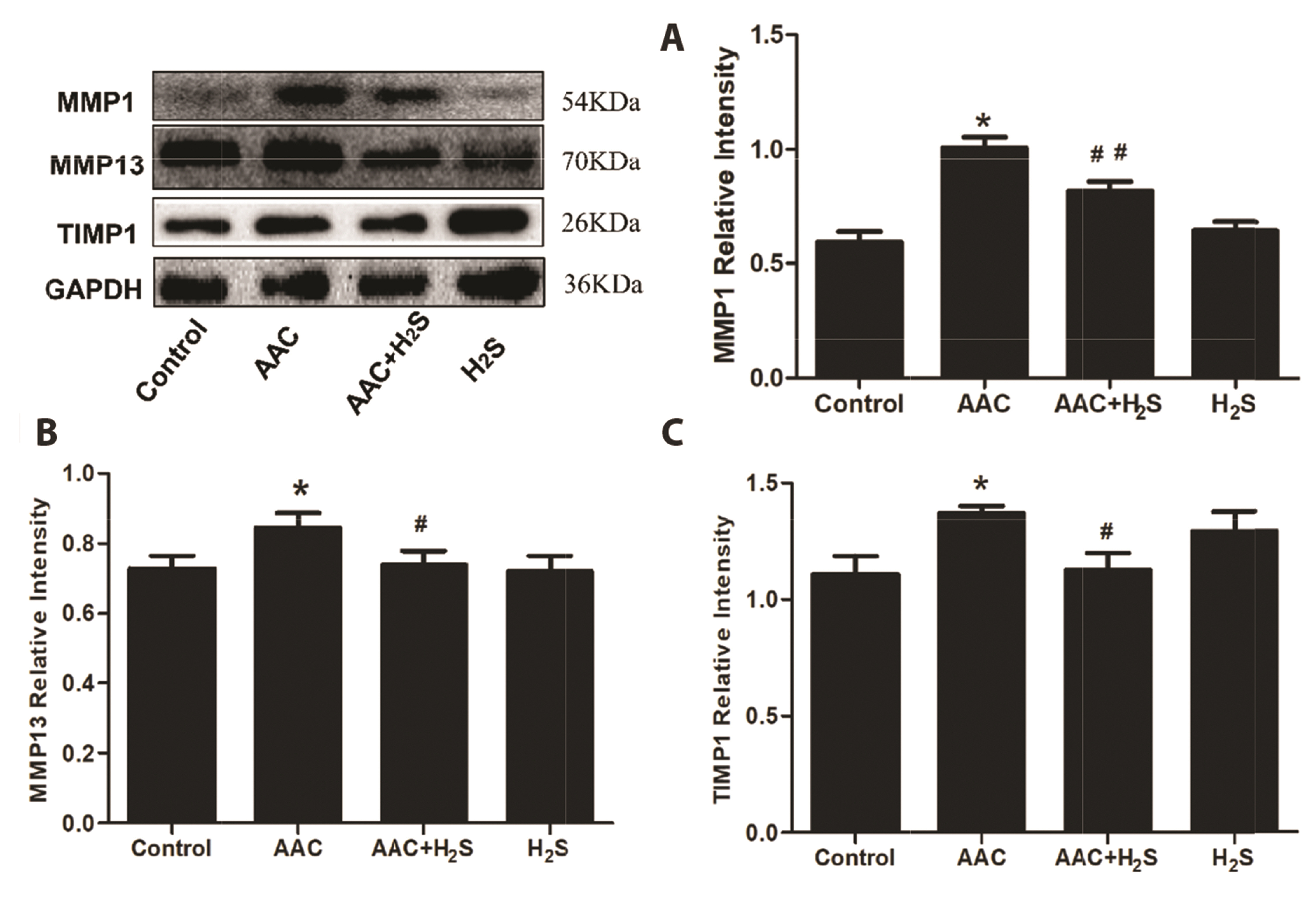
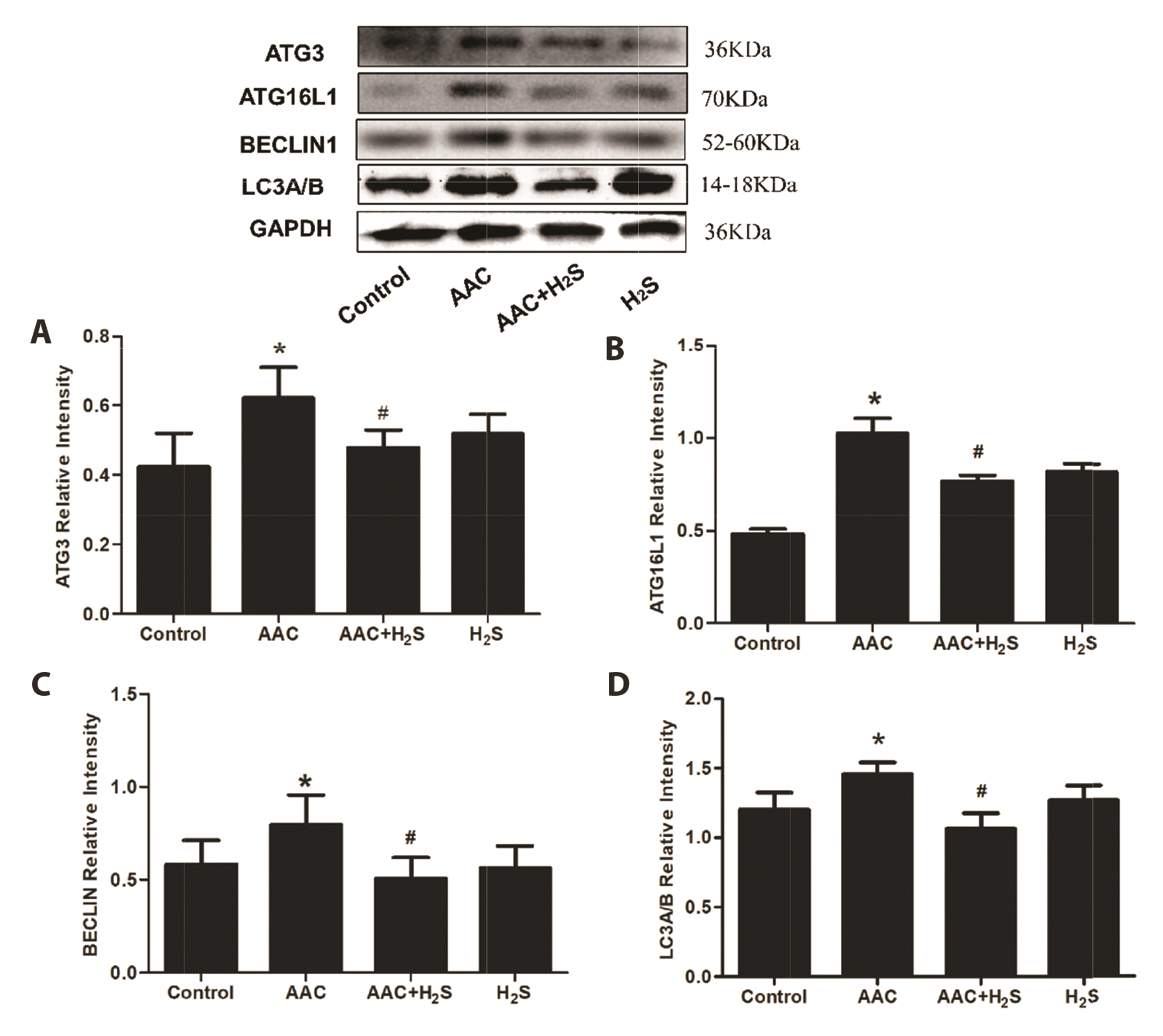
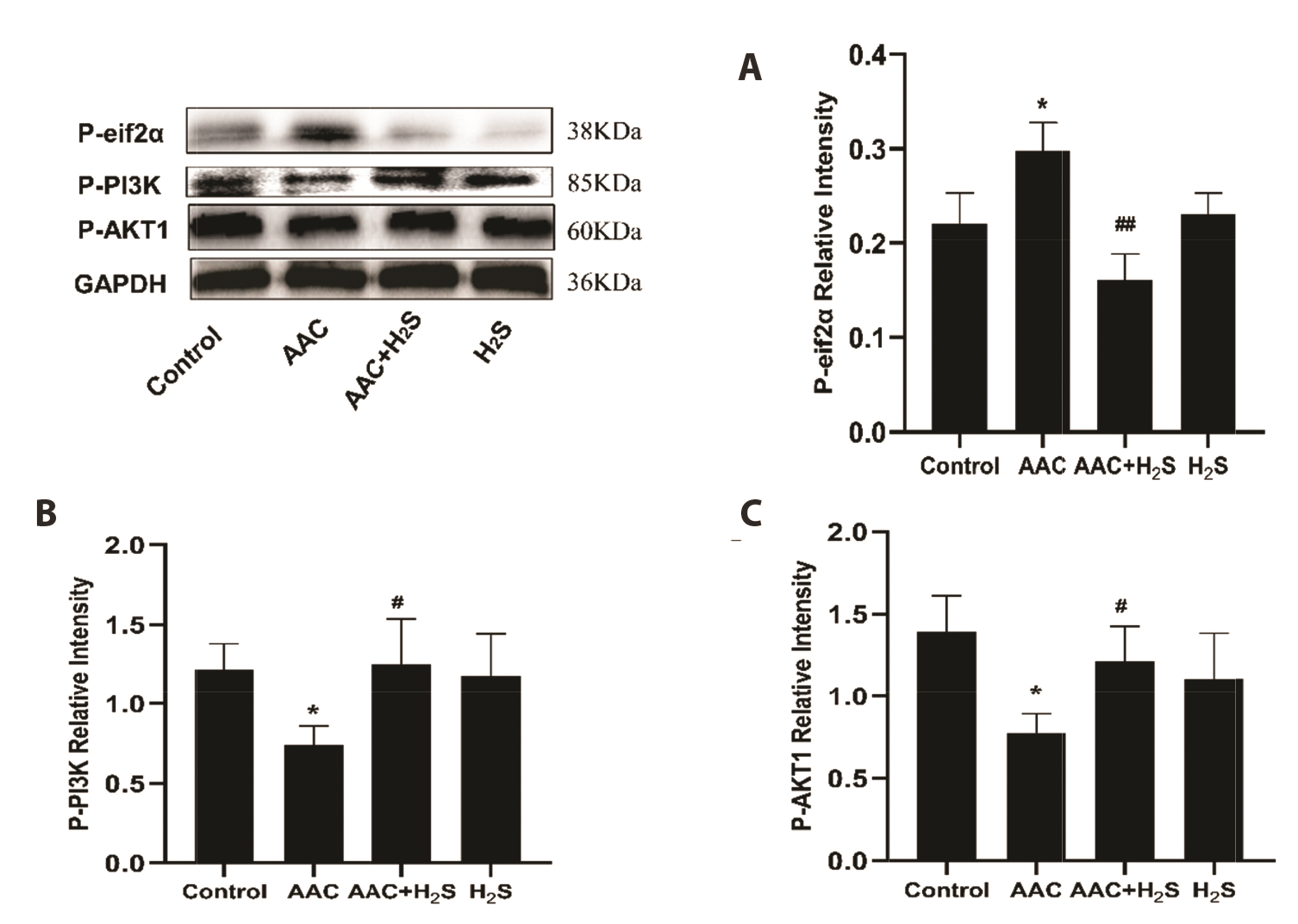
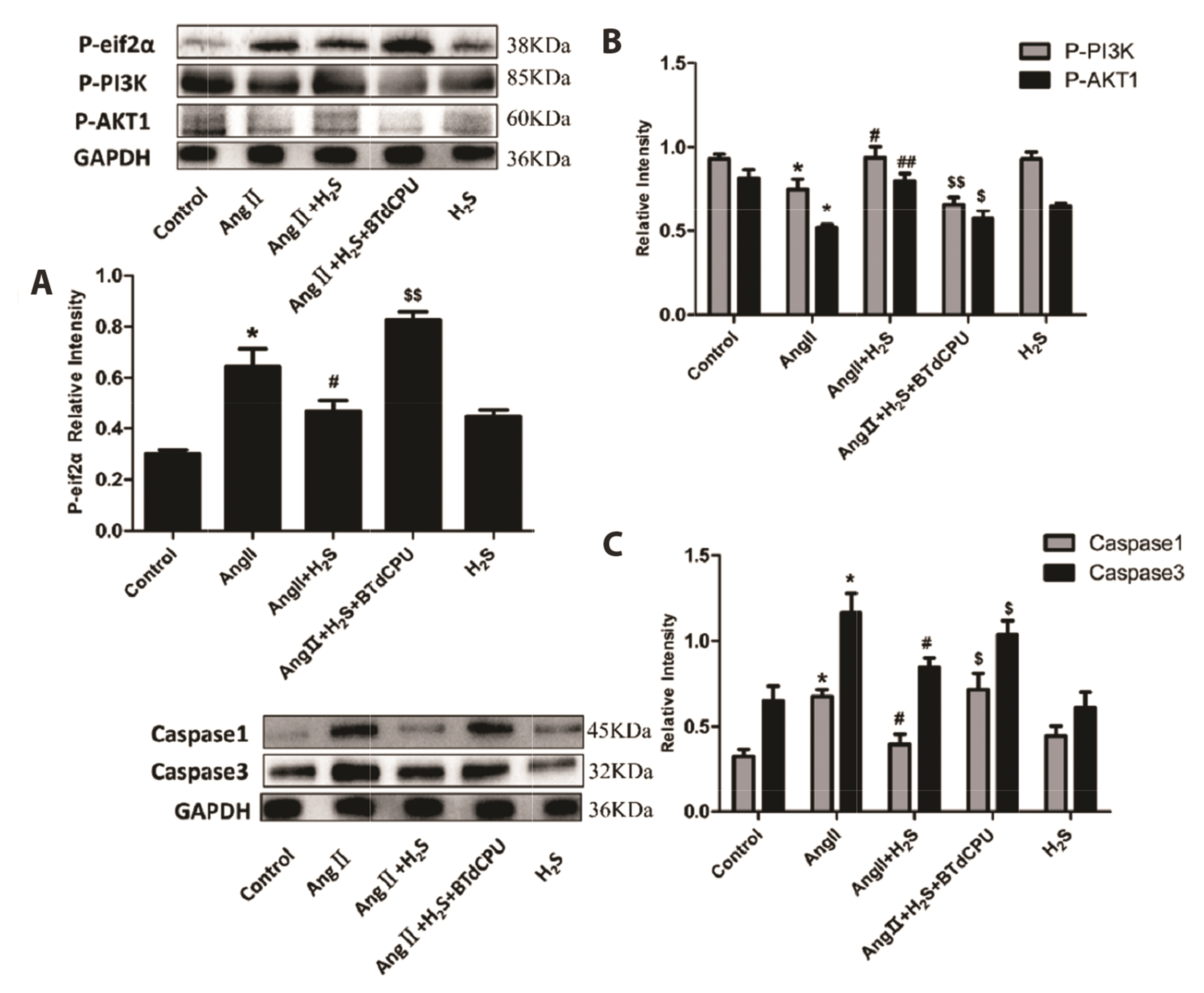
- This study aimed to assess the effects of exogenous hydrogen sulfide (H2S) on abdominal aorta coarctation (AAC) induced myocardial fibrosis (MF) and autophagy in rats. Forty-four Sprague–Dawley rats were randomly divided into control group, AAC group, AAC + H2S group, and H2S control group. After a model of rats with AAC was built surgically, AAC + H2S group and H2S group were injected intraperitoneally with H2S (100 μmol/kg) daily. The rats in the control group and the AAC group were injected with the same amount of PBS. We observed that H2S can improve left ventricular function and the deposition of myocardial collagen fibers, inhibit pyroptosis, down-regulate the expression of P-eif2α in myocardial tissue, and inhibit cell autophagy by activating the phosphatidylinositol 3-kinase (PI3K)/AKT1 signaling pathway (p < 0.05). In addition, angiotensin II (1 μM) H9c2 cardiomyocytes were injured in vitro experiments, and it was also observed that pyroptosis was inhibited after H2S (400 μmol/kg) intervention, the expression of P-eif2α in cardiomyocytes was significantly down-regulated, and the PI3K/AKT1 signaling pathway was activated at the same time. Therefore, increasing the expression of P-eif2α reverses the activation of the PI3K/AKT1 signaling pathway by H2S. In conclusion, these findings suggest that exogenous H2S can ameliorate MF in rats with AAC by inhibiting pyroptosis, and the mechanism may be associated with inhibiting the phosphorylation of eif2α and activating the PI3K/AKT1 signaling pathway to inhibit excessive cell autophagy.
Figure
Reference
-
1. Berk BC, Fujiwara K, Lehoux S. 2007; ECM remodeling in hypertensive heart disease. J Clin Invest. 117:568–575. DOI: 10.1172/JCI31044. PMID: 17332884. PMCID: PMC1804378.
Article2. Li J, Xue J, Wang D, Dai X, Sun Q, Xiao T, Wu L, Xia H, Mostofa G, Chen X, Wei Y, Chen F, Quamruzzaman Q, Zhang A, Liu Q. 2019; Regulation of gasdermin D by miR-379-5p is involved in arsenite-induced activation of hepatic stellate cells and in fibrosis via secretion of IL-1β from human hepatic cells. Metallomics. 11:483–495. DOI: 10.1039/C8MT00321A. PMID: 30643918.
Article3. Chou X, Ding F, Zhang X, Ding X, Gao H, Wu Q. 2019; Sirtuin-1 ameliorates cadmium-induced endoplasmic reticulum stress and pyroptosis through XBP-1s deacetylation in human renal tubular epithelial cells. Arch Toxicol. 93:965–986. DOI: 10.1007/s00204-019-02415-8. PMID: 30796460.
Article4. Wree A, Eguchi A, McGeough MD, Pena CA, Johnson CD, Canbay A, Hoffman HM, Feldstein AE. 2014; NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology. 59:898–910. DOI: 10.1002/hep.26592. PMID: 23813842. PMCID: PMC4008151.
Article5. Zhaolin Z, Guohua L, Shiyuan W, Zuo W. 2019; Role of pyroptosis in cardiovascular disease. Cell Prolif. 52:e12563. DOI: 10.1111/cpr.12563. PMID: 30525268. PMCID: PMC6496801.
Article6. Asano J, Sato T, Ichinose S, Kajita M, Onai N, Shimizu S, Ohteki T. 2017; Intrinsic autophagy is required for the maintenance of intestinal stem cells and for irradiation-induced intestinal regeneration. Cell Rep. 20:1050–1060. DOI: 10.1016/j.celrep.2017.07.019. PMID: 28768191.
Article7. Terman A, Brunk UT. 2006; Oxidative stress, accumulation of biological 'garbage', and aging. Antioxid Redox Signal. 8:197–204. DOI: 10.1089/ars.2006.8.197. PMID: 16487053.
Article8. Lo SH, Hsu CT, Niu HS, Niu CS, Cheng JT, Chen ZC. 2017; Cryptotanshinone inhibits STAT3 signaling to alleviate cardiac fibrosis in type 1-like diabetic rats. Phytother Res. 31:638–646. DOI: 10.1002/ptr.5777. PMID: 28176375.
Article9. Aoki M, Fujishita T. 2017; Oncogenic roles of the PI3K/AKT/mTOR axis. Curr Top Microbiol Immunol. 407:153–189. DOI: 10.1007/82_2017_6. PMID: 28550454.
Article10. Xiao T, Luo J, Wu Z, Li F, Zeng O, Yang J. 2016; Effects of hydrogen sulfide on myocardial fibrosis and PI3K/AKT1-regulated autophagy in diabetic rats. Mol Med Rep. 13:1765–1773. DOI: 10.3892/mmr.2015.4689. PMID: 26676365.
Article11. Li Z, Zhao F, Cao Y, Zhang J, Shi P, Sun X, Zhang F, Tong L. 2018; DHA attenuates hepatic ischemia reperfusion injury by inhibiting pyroptosis and activating PI3K/Akt pathway. Eur J Pharmacol. 835:1–10. DOI: 10.1016/j.ejphar.2018.07.054. PMID: 30075219.
Article12. Liu Y, Tie L. 2019; Apolipoprotein M and sphingosine-1-phosphate complex alleviates TNF-α-induced endothelial cell injury and inflammation through PI3K/AKT signaling pathway. BMC Cardiovasc Disord. 19:279. DOI: 10.1186/s12872-019-1263-4. PMID: 31791242. PMCID: PMC6889454. PMID: 5701fd294d124fe69776342c0f6888b4.
Article13. Moon SL, Sonenberg N, Parker R. 2018; Neuronal regulation of eIF2α function in health and neurological disorders. Trends Mol Med. 24:575–589. DOI: 10.1016/j.molmed.2018.04.001. PMID: 29716790.
Article14. Kazemi S, Mounir Z, Baltzis D, Raven JF, Wang S, Krishnamoorthy JL, Pluquet O, Pelletier J, Koromilas AE. 2007; A novel function of eIF2alpha kinases as inducers of the phosphoinositide-3 kinase signaling pathway. Mol Biol Cell. 18:3635–3644. DOI: 10.1091/mbc.e07-01-0053. PMID: 17596516. PMCID: PMC1951772.
Article15. Koromilas AE, Mounir Z. 2013; Control of oncogenesis by eIF2α phosphorylation: implications in PTEN and PI3K-Akt signaling and tumor treatment. Future Oncol. 9:1005–1015. DOI: 10.2217/fon.13.49. PMID: 23837763.
Article16. Calvert JW, Coetzee WA, Lefer DJ. 2010; Novel insights into hydrogen sulfide--mediated cytoprotection. Antioxid Redox Signal. 12:1203–1217. DOI: 10.1089/ars.2009.2882. PMID: 19769484. PMCID: PMC2864658.
Article17. Chen K, Rekep M, Wei W, Wu Q, Xue Q, Li S, Tian J, Yi Q, Zhang G, Zhang G, Xiao Q, Luo J, Liu Y. 2018; Quercetin prevents in vivo and in vitro myocardial hypertrophy through the proteasome-GSK-3 pathway. Cardiovasc Drugs Ther. 32:5–21. DOI: 10.1007/s10557-018-6771-4. PMID: 29435775.
Article18. Burwick N, Zhang MY, de la Puente P, Azab AK, Hyun TS, Ruiz-Gutierrez M, Sanchez-Bonilla M, Nakamura T, Delrow JJ, MacKay VL, Shimamura A. 2017; The eIF2-alpha kinase HRI is a novel therapeutic target in multiple myeloma. Leuk Res. 55:23–32. DOI: 10.1016/j.leukres.2017.01.007. PMID: 28119225. PMCID: PMC5354961.
Article19. Bing OH. 1994; Hypothesis: apoptosis may be a mechanism for the transition to heart failure with chronic pressure overload. J Mol Cell Cardiol. 26:943–948. DOI: 10.1006/jmcc.1994.1115. PMID: 7799449.
Article20. Heusch G, Libby P, Gersh B, Yellon D, Böhm M, Lopaschuk G, Opie L. 2014; Cardiovascular remodelling in coronary artery disease and heart failure. Lancet. 383:1933–1943. DOI: 10.1016/S0140-6736(14)60107-0. PMID: 24831770.
Article21. Pei Z, Meng R, Li G, Yan G, Xu C, Zhuang Z, Ren J, Wu Z. 2010; Angiotensin-(1-7) ameliorates myocardial remodeling and interstitial fibrosis in spontaneous hypertension: role of MMPs/TIMPs. Toxicol Lett. 199:173–181. DOI: 10.1016/j.toxlet.2010.08.021. PMID: 20837116.
Article22. Nagase H, Visse R, Murphy G. 2006; Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 69:562–573. DOI: 10.1016/j.cardiores.2005.12.002. PMID: 16405877.
Article23. Xu YJ, Zheng L, Hu YW, Wang Q. 2018; Pyroptosis and its relationship to atherosclerosis. Clin Chim Acta. 476:28–37. DOI: 10.1016/j.cca.2017.11.005. PMID: 29129476.
Article24. Frank D, Vince JE. 2019; Pyroptosis versus necroptosis: similarities, differences, and crosstalk. Cell Death Differ. 26:99–114. DOI: 10.1038/s41418-018-0212-6. PMID: 30341423. PMCID: PMC6294779.
Article25. Beier JI, Banales JM. 2018; Pyroptosis: an inflammatory link between NAFLD and NASH with potential therapeutic implications. J Hepatol. 68:643–645. DOI: 10.1016/j.jhep.2018.01.017. PMID: 29408544. PMCID: PMC6185810.
Article26. Qiu T, Pei P, Yao X, Jiang L, Wei S, Wang Z, Bai J, Yang G, Gao N, Yang L, Qi S, Yan R, Liu X, Sun X. 2018; Taurine attenuates arsenic-induced pyroptosis and nonalcoholic steatohepatitis by inhibiting the autophagic-inflammasomal pathway. Cell Death Dis. 9:946. DOI: 10.1038/s41419-018-1004-0. PMID: 30237538. PMCID: PMC6148242.
Article27. Jiang C, Jiang L, Li Q, Liu X, Zhang T, Dong L, Liu T, Liu L, Hu G, Sun X, Jiang L. 2018; Acrolein induces NLRP3 inflammasome-mediated pyroptosis and suppresses migration via ROS-dependent autophagy in vascular endothelial cells. Toxicology. 410:26–40. DOI: 10.1016/j.tox.2018.09.002. PMID: 30205151.
Article28. Wek RC. 2018; Role of eIF2α kinases in translational control and adaptation to cellular stress. Cold Spring Harb Perspect Biol. 10:a032870. DOI: 10.1101/cshperspect.a032870. PMID: 29440070. PMCID: PMC6028073.
Article29. Pakos-Zebrucka K, Koryga I, Mnich K, Ljujic M, Samali A, Gorman AM. 2016; The integrated stress response. EMBO Rep. 17:1374–1395. DOI: 10.15252/embr.201642195. PMID: 27629041. PMCID: PMC5048378.
Article30. Ron D, Walter P. 2007; Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 8:519–529. DOI: 10.1038/nrm2199. PMID: 17565364.
Article31. Sano R, Reed JC. 2013; ER stress-induced cell death mechanisms. Biochim Biophys Acta. 1833:3460–3470. DOI: 10.1016/j.bbamcr.2013.06.028. PMID: 23850759. PMCID: PMC3834229.
Article32. Huo JY, Jiang WY, Geng J, Chen C, Zhu L, Chen R, Ge TT, Chang Q, Jiang ZX, Shan QJ. 2019; Renal denervation attenuates pressure overload-induced cardiac remodelling in rats with biphasic regulation of autophagy. Acta Physiol (Oxf). 226:e13272. DOI: 10.1111/apha.13272. PMID: 30830723.
Article33. Zheng SF, Bao RK, Zhang QJ, Wang SC, Lin HJ. 2018; Endogenous hydrogen sulfide promotes apoptosis via mitochondrial pathways in the livers of broilers with selenium deficiency exudative diathesis disease. Biol Trace Elem Res. 186:249–257. DOI: 10.1007/s12011-018-1292-3. PMID: 29524194.
Article34. Prabhudesai S, Koceja C, Dey A, Eisa-Beygi S, Leigh NR, Bhattacharya R, Mukherjee P, Ramchandran R. 2018; Corrigendum: Cystathionine β-synthase is necessary for axis development in vivo. Front Cell Dev Biol. 6:121. Erratum for: Front Cell Dev Biol. 2018;6:14. DOI: 10.3389/fcell.2018.00121. PMID: 30310813. PMCID: PMC6171515. PMID: 51d1173a673543668a44fc02def0c0f6.
Article35. Long J, Liu M, Liu S, Tang F, Tan W, Xiao T, Chu C, Yang J. 2019; H2S attenuates the myocardial fibrosis in diabetic rats through modulating PKC-ERK1/2MAPK signaling pathway. Technol Health Care. 27(S1):307–316. DOI: 10.3233/THC-199029. PMID: 31045549. PMCID: PMC6598001.
Article36. Predmore BL, Lefer DJ, Gojon G. 2012; Hydrogen sulfide in biochemistry and medicine. Antioxid Redox Signal. 17:119–140. DOI: 10.1089/ars.2012.4612. PMID: 22432697. PMCID: PMC3342665.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hydrogen sulfide alleviates hypothyroidism-induced myocardial fibrosis in rats through stimulating autophagy and inhibiting TGF-β1/Smad2 pathway
- The Heterochromatin-1 Phosphorylation Contributes to TPA-Induced AP-1 Expression
- Translational Regulation: A Novel Target for Breast Cancer Therapy
- Isolation of hydrogen sulfide producing escherichia coli
- Serial MRI of Hypoxic Brain Damage after Hydrogen Sulfide Exposure