J Lipid Atheroscler.
2020 Jan;9(1):110-123. 10.12997/jla.2020.9.1.110.
Quantifications of Lipid Kinetics In Vivo Using Stable Isotope Tracer Methodology
- Affiliations
-
- 1Department of Molecular Medicine, Lee Gil Ya Cancer and Diabetes Institute, College of Medicine, Gachon University, Incheon, Korea. iykim@gachon.ac.kr
- 2Department of Geriatrics, Center for Translational Research in Aging & Longevity, Donald W. Reynolds Institute on Aging, University of Arkansas for Medical Sciences, Little Rock, AR, USA.
- KMID: 2470804
- DOI: http://doi.org/10.12997/jla.2020.9.1.110
Abstract
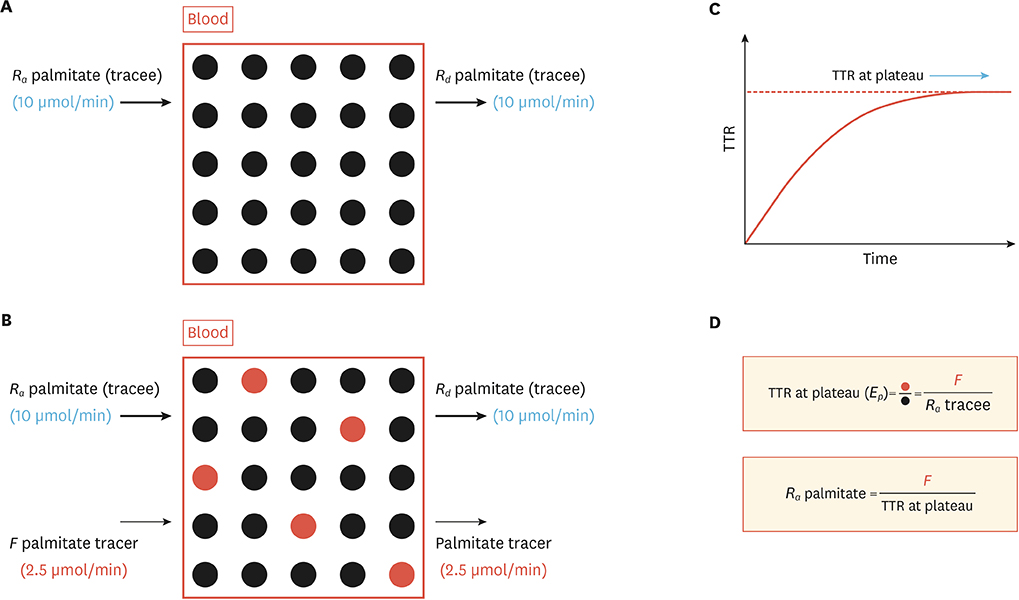
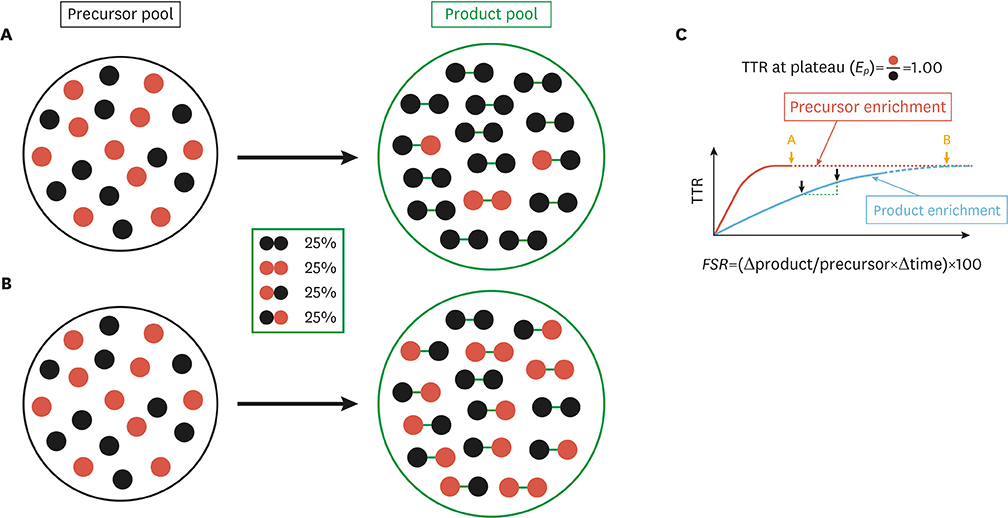
- Like other bodily materials, lipids such as plasma triacylglycerol, cholesterols, and free fatty acids are in a dynamic state of constant turnover (i.e., synthesis, breakdown, oxidation, and/or conversion to other compounds) as essential processes for achieving dynamic homeostasis in the body. However, dysregulation of lipid turnover can lead to clinical conditions such as obesity, fatty liver disease, and dyslipidemia. Assessment of "snap-shot" information on lipid metabolism (e.g., tissue contents of lipids, abundance of mRNA and protein and/or signaling molecules) are often used in clinical and research settings, and can help to understand one's health and disease status. However, such "snapshots" do not provide critical information on dynamic nature of lipid metabolism, and therefore may miss "true" origin of the dysregulation implicated in related diseases. In this regard, stable isotope tracer methodology can provide the in vivo kinetic information of lipid metabolism. Combining with "static" information, knowledge of lipid kinetics can enable the acquisition of in depth understanding of lipid metabolism in relation to various health and disease status. This in turn facilitates the development of effective therapeutic approaches (e.g., exercise, nutrition, and/or drugs). In this review we will discuss 1) the importance of obtaining kinetic information for a better understanding of lipid metabolism, 2) basic principles of stable isotope tracer methodologies that enable exploration of "lipid kinetics" in vivo, and 3) quantification of some aspects of lipid kinetics in vivo with numerical examples.
MeSH Terms
Figure
Cited by 1 articles
-
In Vivo andIn Vitro Quantification of Glucose Kinetics: From Bedside to Bench
Il-Young Kim, Sanghee Park, Yeongmin Kim, Yewon Chang, Cheol Soo Choi, Sang-Hoon Suh, Robert R. Wolfe
Endocrinol Metab. 2020;35(4):733-749. doi: 10.3803/EnM.2020.406.
Reference
-
1. Schoenheimer R. The dynamic state of body constituents. Cambridge (MA): Harvard University Express;1946.2. Schoenheimer R, Rittenberg D. Deuterium as an indicator in the study of intermediary metabolism: VI. Synthesis and destruction of fatty acids in the organism. J Biol Chem. 1937; 114:381–396.3. Rittenberg D, Schoenheimer R. Deuterium as an indicator in the study of intermediary metabolism: XI. Further studies on the biological uptake of deuterium into organic substances, with special reference to fat and cholesterol formation. J Biol Chem. 1937; 121:235–253.4. Kim IY, Park S, Trombold JR, Coyle EF. Effects of moderate- and intermittent low-intensity exercise on postprandial lipemia. Med Sci Sports Exerc. 2014; 46:1882–1890.
Article5. Kim IY, Park S, Chou TH, Trombold JR, Coyle EF. Prolonged sitting negatively affects the postprandial plasma triglyceride-lowering effect of acute exercise. Am J Physiol Endocrinol Metab. 2016; 311:E891–E898.
Article6. Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007; 298:309–316.
Article7. Bukhari SS, Phillips BE, Wilkinson DJ, Limb MC, Rankin D, Mitchell WK, et al. Intake of low-dose leucine-rich essential amino acids stimulates muscle anabolism equivalently to bolus whey protein in older women at rest and after exercise. Am J Physiol Endocrinol Metab. 2015; 308:E1056–E1065.
Article8. Perry RJ, Wang Y, Cline GW, Rabin-Court A, Song JD, Dufour S, et al. Leptin mediates a glucose-fatty acid cycle to maintain glucose homeostasis in starvation. Cell. 2018; 172:234–248.e17.
Article9. Neinast MD, Jang C, Hui S, Murashige DS, Chu Q, Morscher RJ, et al. Quantitative analysis of the whole-body metabolic fate of branched-chain amino acids. Cell Metab. 2019; 29:417–429.e4.
Article10. Hellerstein MK. In vivo measurement of fluxes through metabolic pathways: the missing link in functional genomics and pharmaceutical research. Annu Rev Nutr. 2003; 23:379–402.
Article11. McGuire EA, Helderman JH, Tobin JD, Andres R, Berman M. Effects of arterial versus venous sampling on analysis of glucose kinetics in man. J Appl Physiol. 1976; 41:565–573.
Article12. Goodpaster BH, Wolfe RR, Kelley DE. Effects of obesity on substrate utilization during exercise. Obes Res. 2002; 10:575–584.
Article13. Kim IY, Williams RH, Schutzler SE, Lasley CJ, Bodenner DL, Wolfe RR, et al. Acute lysine supplementation does not improve hepatic or peripheral insulin sensitivity in older, overweight individuals. Nutr Metab (Lond). 2014; 11:49.
Article14. Kim IY, Schutzler SE, Azhar G, Wolfe RR, Ferrando AA, Coker RH. Short term elevation in dietary protein intake does not worsen insulin resistance or lipids in older adults with metabolic syndrome: a randomized-controlled trial. BMC Nutr. 2017; 3:33.
Article15. Chopra S, Rathore A, Younas H, Pham LV, Gu C, Beselman A, et al. Obstructive sleep apnea dynamically increases nocturnal plasma free fatty acids, glucose, and cortisol during sleep. J Clin Endocrinol Metab. 2017; 102:3172–3181.
Article16. Gjedsted J, Buhl M, Nielsen S, Schmitz O, Vestergaard ET, Tønnesen E, et al. Effects of adrenaline on lactate, glucose, lipid and protein metabolism in the placebo controlled bilaterally perfused human leg. Acta Physiol (Oxf). 2011; 202:641–648.
Article17. Kim IY, Shin YA, Schutzler SE, Azhar G, Wolfe RR, Ferrando AA. Quality of meal protein determines anabolic response in older adults. Clin Nutr. 2018; 37:2076–2083.
Article18. Kim IY, Schutzler S, Schrader A, Spencer HJ, Azhar G, Ferrando AA, et al. The anabolic response to a meal containing different amounts of protein is not limited by the maximal stimulation of protein synthesis in healthy young adults. Am J Physiol Endocrinol Metab. 2016; 310:E73–E80.
Article19. Kim IY, Park S, Smeets ET, Schutzler S, Azhar G, Wei JY, et al. Consumption of a specially-formulated mixture of essential amino acids promotes gain in whole-body protein to a greater extent than a complete meal replacement in older women with heart failure. Nutrients. 2019; 11:E1360.
Article20. Kim IY, Schutzler S, Schrader A, Spencer H, Kortebein P, Deutz NE, et al. Quantity of dietary protein intake, but not pattern of intake, affects net protein balance primarily through differences in protein synthesis in older adults. Am J Physiol Endocrinol Metab. 2015; 308:E21–E28.
Article21. de Betue CT, Joosten KF, Deutz NE, Vreugdenhil AC, van Waardenburg DA. Arginine appearance and nitric oxide synthesis in critically ill infants can be increased with a protein-energy-enriched enteral formula. Am J Clin Nutr. 2013; 98:907–916.
Article22. Castillo L, Beaumier L, Ajami AM, Young VR. Whole body nitric oxide synthesis in healthy men determined from [15N] arginine-to-[15N]citrulline labeling. Proc Natl Acad Sci U S A. 1996; 93:11460–11465.
Article23. Kim IY, Schutzler SE, Schrader A, Spencer HJ, Azhar G, Deutz NE, et al. Acute ingestion of citrulline stimulates nitric oxide synthesis but does not increase blood flow in healthy young and older adults with heart failure. Am J Physiol Endocrinol Metab. 2015; 309:E915–E924.
Article24. Kim IY, Suh SH, Lee IK, Wolfe RR. Applications of stable, nonradioactive isotope tracers in in vivo human metabolic research. Exp Mol Med. 2016; 48:e203.25. Wolfe RR, Chinkes DL. Isotope tracers in metabolic research: principles and practice of kinetic analysis. New York (NY): Wiley-Liss;2004.26. Mazzeo RS, Brooks GA, Schoeller DA, Budinger TF. Disposal of blood [1-13C]lactate in humans during rest and exercise. J Appl Physiol (1985). 1986; 60:232–241.
Article27. Kim IY, Schutzler S, Schrader AM, Spencer HJ, Azhar G, Wolfe RR, et al. Protein intake distribution pattern does not affect anabolic response, lean body mass, muscle strength or function over 8 weeks in older adults: a randomized-controlled trial. Clin Nutr. 2018; 37:488–493.
Article28. Martini WZ, Chinkes DL, Wolfe RR. Quantification of DNA synthesis from different pathways in cultured human fibroblasts and myocytes. Metabolism. 2004; 53:128–133.
Article29. Fulks RM, Li JB, Goldberg AL. Effects of insulin, glucose, and amino acids on protein turnover in rat diaphragm. J Biol Chem. 1975; 250:290–298.
Article30. Zhang XJ, Chinkes DL, Wu Z, Martini WZ, Wolfe RR. Fractional synthesis rates of DNA and protein in rabbit skin are not correlated. J Nutr. 2004; 134:2401–2406.
Article31. Brook MS, Wilkinson DJ, Mitchell WK, Lund JL, Phillips BE, Szewczyk NJ, et al. A novel D2O tracer method to quantify RNA turnover as a biomarker of de novo ribosomal biogenesis, in vitro, in animal models, and in human skeletal muscle. Am J Physiol Endocrinol Metab. 2017; 313:E681–E689.32. Khairallah EA, Mortimore GE. Assessment of protein turnover in perfused rat liver. Evidence for amino acid compartmentation from differential labeling of free and tRNA-gound valine. J Biol Chem. 1976; 251:1375–1384.
Article33. Wolfe RR, Peters EJ, Klein S, Holland OB, Rosenblatt J, Gary H Jr. Effect of short-term fasting on lipolytic responsiveness in normal and obese human subjects. Am J Physiol. 1987; 252:E189–E196.
Article34. Wolfe RR, Peters EJ. Lipolytic response to glucose infusion in human subjects. Am J Physiol. 1987; 252:E218–E223.
Article35. Greenough WB 3rd, Crespin SR, Steinberg D. Infusion of long-chain fatty acid anions by continuous-flow centrifugation. J Clin Invest. 1969; 48:1923–1933.
Article36. Hellerstein MK, Benowitz NL, Neese RA, Schwartz JM, Hoh R, Jacob P 3rd, et al. Effects of cigarette smoking and its cessation on lipid metabolism and energy expenditure in heavy smokers. J Clin Invest. 1994; 93:265–272.
Article37. Fontbonne A, Eschwège E, Cambien F, Richard JL, Ducimetière P, Thibult N, et al. Hypertriglyceridaemia as a risk factor of coronary heart disease mortality in subjects with impaired glucose tolerance or diabetes. Results from the 11-year follow-up of the Paris Prospective Study. Diabetologia. 1989; 32:300–304.
Article38. Roche HM, Gibney MJ. The impact of postprandial lipemia in accelerating atherothrombosis. J Cardiovasc Risk. 2000; 7:317–324.
Article39. Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005; 115:1343–1351.
Article40. Sidossis LS, Coggan AR, Gastaldelli A, Wolfe RR. Pathway of free fatty acid oxidation in human subjects. Implications for tracer studies. J Clin Invest. 1995; 95:278–284.
Article41. Turner SM, Murphy EJ, Neese RA, Antelo F, Thomas T, Agarwal A, et al. Measurement of TG synthesis and turnover in vivo by 2H2O incorporation into the glycerol moiety and application of MIDA. Am J Physiol Endocrinol Metab. 2003; 285:E790–E803.
Article42. Cobelli C, Foster D, Toffolo G. Tracer kinetics in biomedical research. Boston (MA): Springer US;2002.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Applications of stable, nonradioactive isotope tracers in in vivo human metabolic research
- Exploring Human Muscle Dynamics In Vivo Using Stable Isotope Tracers
- In Vivo and In Vitro Quantification of Glucose Kinetics: From Bedside to Bench
- The association between carbon and nitrogen stable isotope ratios of human hair and hypertension
- Stable isotope analysis of Joseon people skeletons from the cemeteries of Old Seoul City, the capital of Joseon Dynasty