Korean J Physiol Pharmacol.
2020 Jan;24(1):121-126. 10.4196/kjpp.2020.24.1.121.
Ezrin-radixin-moesin proteins are regulated by Akt-GSK3β signaling in the rat nucleus accumbens core
- Affiliations
-
- 1Department of Physiology, Brain Korea 21 Plus Project for Medical Science, Yonsei University College of Medicine, Seoul 03722, Korea. jkim1@yuhs.ac
- 2Department of Medical Science, Brain Korea 21 Plus Project for Medical Science, Yonsei University College of Medicine, Seoul 03722, Korea.
- 3Bio-Pharm Solutions Co., Ltd., Suwon 16229, Korea.
- KMID: 2466576
- DOI: http://doi.org/10.4196/kjpp.2020.24.1.121
Abstract
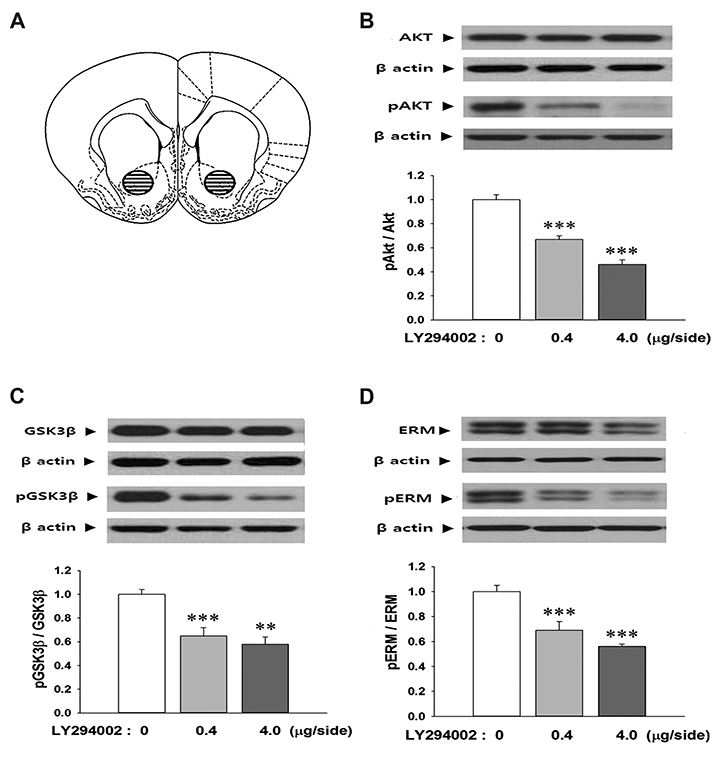
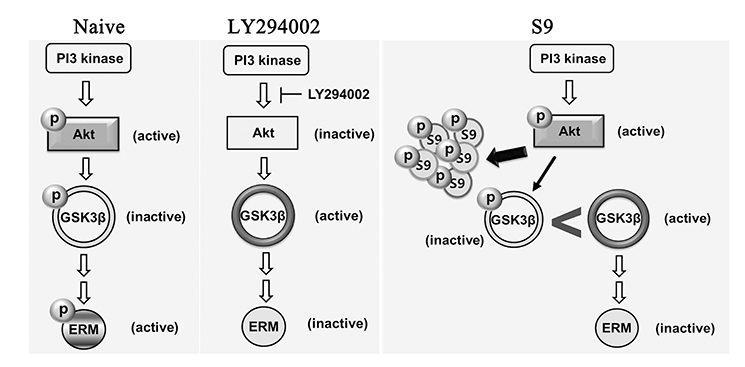
- The ezrin-radixin-moesin (ERM) proteins are a family of membrane-associated proteins known to play roles in cell-shape determination as well as in signaling pathways. We have previously shown that amphetamine decreases phosphorylation levels of these proteins in the nucleus accumbens (NAcc), an important neuronal substrate mediating rewarding effects of drugs of abuse. In the present study, we further examined what molecular pathways may be involved in this process. By direct microinjection of LY294002, a PI3 kinase inhibitor, or of S9 peptide, a proposed GSK3β activator, into the NAcc core, we found that phosphorylation levels of ERM as well as of GSK3β in this site are simultaneously decreased. These results indicate that ERM proteins are under the regulation of Akt-GSK3β signaling pathway in the NAcc core. The present findings have a significant implication to a novel signal pathway possibly leading to structural plasticity in relation with drug addiction.
Keyword
MeSH Terms
-
Amphetamine
Animals
Glycogen Synthase Kinases
Humans
Membrane Proteins
Microinjections
Negotiating
Neurons
Nucleus Accumbens*
Phosphorylation
Phosphotransferases
Plastics
Proto-Oncogene Proteins c-akt
Rats*
Reward
Signal Transduction
Street Drugs
Substance-Related Disorders
Amphetamine
Glycogen Synthase Kinases
Membrane Proteins
Phosphotransferases
Plastics
Proto-Oncogene Proteins c-akt
Street Drugs
Figure
Cited by 1 articles
-
Decrease of glycogen synthase kinase 3β phosphorylation in the rat nucleus accumbens shell is necessary for amphetamine-induced conditioned locomotor activity
Joong-Keun Shin, Wha Young Kim, Haeun Rim, Jeong-Hoon Kim
Korean J Physiol Pharmacol. 2022;26(1):59-65. doi: 10.4196/kjpp.2022.26.1.59.
Reference
-
1. Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002; 3:586–599.
Article2. Louvet-Vallée S. ERM proteins: from cellular architecture to cell signaling. Biol Cell. 2000; 92:305–316.
Article3. Niggli V, Rossy J. Ezrin/radixin/moesin: versatile controllers of signaling molecules and of the cortical cytoskeleton. Int J Biochem Cell Biol. 2008; 40:344–349.
Article4. Pelaseyed T, Bretscher A. Regulation of actin-based apical structures on epithelial cells. J Cell Sci. 2018; 131:jcs221853.
Article5. Matus A. Growth of dendritic spines: a continuing story. Curr Opin Neurobiol. 2005; 15:67–72.
Article6. Neisch AL, Fehon RG. Ezrin, Radixin and Moesin: key regulators of membrane-cortex interactions and signaling. Curr Opin Cell Biol. 2011; 23:377–382.
Article7. Robbins TW, Cador M, Taylor JR, Everitt BJ. Limbic-striatal interactions in reward-related processes. Neurosci Biobehav Rev. 1989; 13:155–162.
Article8. Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001; 24:97–129.
Article9. Goto Y, Grace AA. Limbic and cortical information processing in the nucleus accumbens. Trends Neurosci. 2008; 31:552–558.
Article10. Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005; 8:1445–1449.
Article11. Kim WY, Shin SR, Kim S, Jeon S, Kim JH. Cocaine regulates ezrinradixin-moesin proteins and RhoA signaling in the nucleus accumbens. Neuroscience. 2009; 163:501–505.
Article12. Kim WY, Jang JK, Shin JK, Kim JH. Amphetamine dephosphorylates ERM proteins in the nucleus accumbens core and lithium attenuates its effects. Neurosci Lett. 2013; 552:103–107.
Article13. Beaulieu JM, Caron MG. Looking at lithium: molecular moods and complex behaviour. Mol Interv. 2008; 8:230–241.
Article14. Beaulieu JM, Del'guidice T, Sotnikova TD, Lemasson M, Gainetdinov RR. Beyond cAMP: The regulation of Akt and GSK3 by dopamine receptors. Front Mol Neurosci. 2011; 4:38.
Article15. Li B, Ren J, Yang L, Li X, Sun G, Xia M. Lithium inhibits GSK3β activity via two different signaling pathways in neurons after spinal cord injury. Neurochem Res. 2018; 43:848–856.
Article16. Gallo G. Semaphorin 3A inhibits ERM protein phosphorylation in growth cone filopodia through inactivation of PI3K. Dev Neurobiol. 2008; 68:926–933.
Article17. Jeon S, Park JK, Bae CD, Park J. NGF-induced moesin phosphorylation is mediated by the PI3K, Rac1 and Akt and required for neurite formation in PC12 cells. Neurochem Int. 2010; 56:810–818.
Article18. Cencetti F, Bernacchioni C, Bruno M, Squecco R, Idrizaj E, Berbeglia M, Bruni P, Donati C. Sphingosine 1-phosphate-mediated activation of ezrin-radixin-moesin proteins contributes to cytoskeletal remodeling and changes of membrane properties in epithelial otic vesicle progenitors. Biochim Biophys Acta Mol Cell Res. 2019; 1866:554–565.
Article19. Choi JM, Ahn MH, Chae WJ, Jung YG, Park JC, Song HM, Kim YE, Shin JA, Park CS, Park JW, Park TK, Lee JH, Seo BF, Kim KD, Kim ES, Lee DH, Lee SK, Lee SK. Intranasal delivery of the cytoplasmic domain of CTLA-4 using a novel protein transduction domain prevents allergic inflammation. Nat Med. 2006; 12:574–579.
Article20. Dajani R, Fraser E, Roe SM, Young N, Good V, Dale TC, Pearl LH. Crystal structure of glycogen synthase kinase 3 beta: structural basis for phosphate-primed substrate specificity and autoinhibition. Cell. 2001; 105:721–732.21. Frame S, Cohen P, Biondi RM. A common phosphate binding site explains the unique substrate specificity of GSK3 and its inactivation by phosphorylation. Mol Cell. 2001; 7:1321–1327.
Article22. Pellegrino LJ, Pellegrino AS, Cushman AJ. A stereotaxic atlas of the rat brain. New York: Plenum;1979.23. Kim WY, Jang JK, Lee JW, Jang H, Kim JH. Decrease of GSK3β phosphorylation in the rat nucleus accumbens core enhances cocaine-induced hyper-locomotor activity. J Neurochem. 2013; 125:642–648.
Article24. O'Donnell KC, Gould TD. The behavioral actions of lithium in rodent models: leads to develop novel therapeutics. Neurosci Biobehav Rev. 2007; 31:932–962.25. Jin EJ, Ko HR, Hwang I, Kim BS, Choi JY, Park KW, Cho SW, Ahn JY. Akt regulates neurite growth by phosphorylation-dependent inhibition of radixin proteasomal degradation. Sci Rep. 2018; 8:2557.
Article26. Jeong HJ, Kim JH, Jeon S. Amphetamine-induced ERM proteins phosphorylation is through PKCβ activation in PC12 cells. Korean J Physiol Pharmacol. 2011; 15:245–249.
Article27. Shen HW, Toda S, Moussawi K, Bouknight A, Zahm DS, Kalivas PW. Altered dendritic spine plasticity in cocaine-withdrawn rats. J Neurosci. 2009; 29:2876–2884.
Article28. Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci. 2008; 9:387–399.
Article29. Li Y, Acerbo MJ, Robinson TE. The induction of behavioural sensitization is associated with cocaine-induced structural plasticity in the core (but not shell) of the nucleus accumbens. Eur J Neurosci. 2004; 20:1647–1654.
Article30. Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004; 47 Suppl 1:33–46.
Article31. Lee KW, Kim Y, Kim AM, Helmin K, Nairn AC, Greengard P. Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. Proc Natl Acad Sci U S A. 2006; 103:3399–3404.
Article32. Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5th ed. London: Elsevier Academic;2004.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Amphetamine-induced ERM Proteins Phosphorylation Is through PKCbeta Activation in PC12 Cells
- Ezrin is an Essential Marker for Metastasis of Gynecologic Cancer
- Clinicopathologic Implication of Ezrin Expression in Non-small Cell Lung Cancer
- Uterine cancer and ezrin expression
- Neuroprotection Signaling of Nuclear Akt in Neuronal Cells