J Korean Soc Menopause.
2012 Aug;18(2):81-93.
Ezrin is an Essential Marker for Metastasis of Gynecologic Cancer
- Affiliations
-
- 1Department of Obstetrics and Gynecology, College of Medicine, Soonchunhyang University Cheonan Hospital, Cheonan, Korea. sdchoi@schmc.ac.kr
Abstract
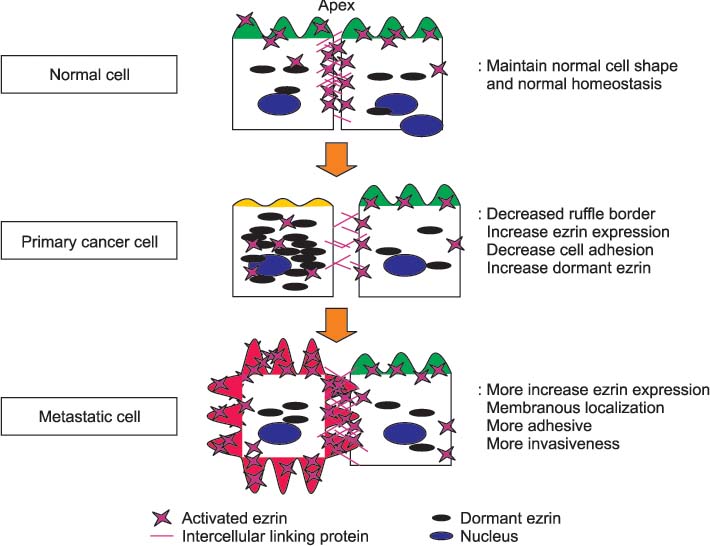
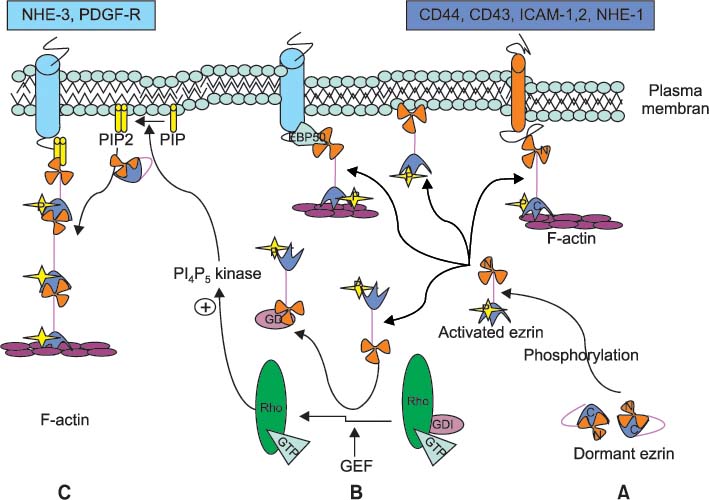
- Ezrin, a membrane cytoskeleton linking protein, is a member of ezrin/radixin/moesin (ERM) that regulates cell shape, motility and cell to cell interaction via linking the contractile elements of the cell to transmembrane proteins. Ezrin, through this mechanism, has been thought to play an important role in cancer progression and distant metastasis. In addition, high levels of ezrin expression have been noted in many cancers, such as breast, colon, osteosarcoma, and prostate cancer. Gynecologic cancer cells, with high levels of ezrin expression, have more invasive potential than that of the lower levels of ezrin expressed cancer cells. High levels of ezrin expression are also related to the advanced histological grade and poor outcome. Recently, several reports have also demonstrated that ezrin expression is enhanced and almost localized at the membranous portion in high stage tumor cells and metastatic gynecologic cancer cells. Therefore, in the near future, ezrin levels and its cellular location might serve as essential markers for the metastasis of gynecologic cancers.
Keyword
MeSH Terms
Figure
Reference
-
1. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005. 55:74–108.2. U.S. Cancer Statistics Working Group. United States Cancer Statistics: 2004 Incidence and Mortality. 2007. Atlanta, U.S: Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute.3. Buda A, Dell'Anna T, Signorelli M, Mangioni C. Role of ifosfamide in cervical cancer: an overview. Oncology. 2003. 65:Suppl 2. 63–66.4. Lee CJ, Ariztia EV, Fishman DA. Conventional and proteomic technologies for the detection of early stage malignancies: markers for ovarian cancer. Crit Rev Clin Lab Sci. 2007. 44:87–114.5. Mehlen P, Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer. 2006. 6:449–458.6. Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002. 3:586–599.7. Curto M, McClatchey AI. Ezrin... a metastatic detERMinant? Cancer Cell. 2004. 5:113–114.8. Martin TA, Harrison G, Mansel RE, Jiang WG. The role of the CD44/ezrin complex in cancer metastasis. Crit Rev Oncol Hematol. 2003. 46:165–186.9. Fazioli F, Wong WT, Ullrich SJ, Sakaguchi K, Appella E, Di Fiore PP. The ezrin-like family of tyrosine kinase substrates: receptor-specific pattern of tyrosine phosphorylation and relationship to malignant transformation. Oncogene. 1993. 8:1335–1345.10. Ohtani K, Sakamoto H, Rutherford T, Chen Z, Kikuchi A, Yamamoto T, et al. Ezrin, a membrane-cytoskeletal linking protein, is highly expressed in atypical endometrial hyperplasia and uterine endometrioid adenocarcinoma. Cancer Lett. 2002. 179:79–86.11. Bruce B, Khanna G, Ren L, Landberg G, Jirström K, Powell C, et al. Expression of the cytoskeleton linker protein ezrin in human cancers. Clin Exp Metastasis. 2007. 24:69–78.12. Chen Z, Fadiel A, Feng Y, Ohtani K, Rutherford T, Naftolin F. Ovarian epithelial carcinoma tyrosine phosphorylation, cell proliferation, and ezrin translocation are stimulated by interleukin 1alpha and epidermal growth factor. Cancer. 2001. 92:3068–3075.13. Köbel M, Langhammer T, Hüttelmaier S, Schmitt WD, Kriese K, Dittmer J, et al. Ezrin expression is related to poor prognosis in FIGO stage I endometrioid carcinomas. Mod Pathol. 2006. 19:581–587.14. Khanna C, Wan X, Bose S, Cassaday R, Olomu O, Mendoza A, et al. The membrane-cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nat Med. 2004. 10:182–186.15. Bretscher A, Gary R, Berryman M. Soluble ezrin purified from placenta exists as stable monomers and elongated dimers with masked C-terminal ezrin-radixin-moesin association domains. Biochemistry. 1995. 34:16830–16837.16. Frixione E. Recurring views on the structure and function of the cytoskeleton: a 300-year epic. Cell Motil Cytoskeleton. 2000. 46:73–94.17. Doherty GJ, McMahon HT. Mediation, modulation, and consequences of membrane-cytoskeleton interactions. Annu Rev Biophys. 2008. 37:65–95.18. Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003. 3:362–374.19. Yonemura S, Tsukita S. Direct involvement of ezrin/radixin/moesin (ERM)-binding membrane proteins in the organization of microvilli in collaboration with activated ERM proteins. J Cell Biol. 1999. 145:1497–1509.20. Lesley J, Hyman R, Kincade PW. CD44 and its interaction with extracellular matrix. Adv Immunol. 1993. 54:271–335.21. Rodríguez-Rodríguez L, Sancho-Torres I, Leakey P, Gibbon DG, Comerci JT, Ludlow JW, et al. CD44 splice variant expression in clear cell carcinoma of the ovary. Gynecol Oncol. 1998. 71:223–229.22. Springer TA. Adhesion receptors of the immune system. Nature. 1990. 346:425–434.23. Arnold JM, Cummings M, Purdie D, Chenevix-Trench G. Reduced expression of intercellular adhesion molecule-1 in ovarian adenocarcinomas. Br J Cancer. 2001. 85:1351–1358.24. Helander TS, Carpén O, Turunen O, Kovanen PE, Vaheri A, Timonen T. ICAM-2 redistributed by ezrin as a target for killer cells. Nature. 1996. 382:265–268.25. Reczek D, Berryman M, Bretscher A. Identification of EBP50: a PDZ-containing phosphoprotein that associates with members of the ezrin-radixin-moesin family. J Cell Biol. 1997. 139:169–179.26. Smith PM, Cowan A, Milgram SL, White BA. Tissue-specific regulation by estrogen of ezrin and ezrin/radixin/moesin-binding protein 50. Endocrine. 2003. 22:119–126.27. Georgescu MM, Morales FC, Molina JR, Hayashi Y. Roles of NHERF1/EBP50 in cancer. Curr Mol Med. 2008. 8:459–468.28. Crepaldi T, Gautreau A, Comoglio PM, Louvard D, Arpin M. Ezrin is an effector of hepatocyte growth factor-mediated migration and morphogenesis in epithelial cells. J Cell Biol. 1997. 138:423–434.29. Kondo T, Takeuchi K, Doi Y, Yonemura S, Nagata S, Tsukita S. ERM (ezrin/radixin/moesin)-based molecular mechanism of microvillar breakdown at an early stage of apoptosis. J Cell Biol. 1997. 139:749–758.30. Hirao M, Sato N, Kondo T, Yonemura S, Monden M, Sasaki T, et al. Regulation mechanism of ERM (ezrin/radixin/moesin) protein/plasma membrane association: possible involvement of phosphatidylinositol turnover and Rho-dependent signaling pathway. J Cell Biol. 1996. 135:37–51.31. Barret C, Roy C, Montcourrier P, Mangeat P, Niggli V. Mutagenesis of the phosphatidylinositol 4,5-bisphosphate (PIP(2)) binding site in the NH(2)-terminal domain of ezrin correlates with its altered cellular distribution. J Cell Biol. 2000. 151:1067–1080.32. Karlsson R, Pedersen ED, Wang Z, Brakebusch C. Rho GTPase function in tumorigenesis. Biochim Biophys Acta. 2009. 1796:91–98.33. del Peso L, Hernández-Alcoceba R, Embade N, Carnero A, Esteve P, Paje C, et al. Rho proteins induce metastatic properties in vivo. Oncogene. 1997. 15:3047–3057.34. Tsai MH, Jiang MJ. Rho-kinase-mediated regulation of receptor-agonist-stimulated smooth muscle contraction. Pflugers Arch. 2006. 453:223–232.35. Takahashi K, Sasaki T, Mammoto A, Takaishi K, Kameyama T, Tsukita S, et al. Direct interaction of the Rho GDP dissociation inhibitor with ezrin/radixin/moesin initiates the activation of the Rho small G protein. J Biol Chem. 1997. 272:23371–23375.36. Smith PM, Heinrich CA, Pappas S, Peluso JJ, Cowan A, White BA. Reciprocal regulation by estradiol 17-beta of ezrin and cadherin-catenin complexes in pituitary GH3 cells. Endocrine. 2002. 17:219–228.37. Stemmer-Rachamimov AO, Wiederhold T, Nielsen GP, James M, Pinney-Michalowski D, Roy JE, et al. NHE-RF, a merlin-interacting protein, is primarily expressed in luminal epithelia, proliferative endometrium, and estrogen receptor-positive breast carcinomas. Am J Pathol. 2001. 158:57–62.38. Ohtani K, Sakamoto H, Rutherford T, Chen Z, Satoh K, Naftolin F. Ezrin, a membrane-cytoskeletal linking protein, is involved in the process of invasion of endometrial cancer cells. Cancer Lett. 1999. 147:31–38.39. Song J, Fadiel A, Edusa V, Chen Z, So J, Sakamoto H, et al. Estradiol-induced ezrin overexpression in ovarian cancer: a new signaling domain for estrogen. Cancer Lett. 2005. 220:57–65.40. Weiss L. Metastasis of cancer: a conceptual history from antiquity to the 1990s. Cancer Metastasis Rev. 2000. 19:I–XI. 193–383.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prognostic Significance of Ezrin Expression in Liposarcoma
- Erratum to: Ezrin Is an Essential Marker for Metastasis of Gynecologic Cancer
- Clinicopathologic Implication of Ezrin Expression in Non-small Cell Lung Cancer
- Clinical Value of Ezrin Expression in Primary Osteosarcoma
- Uterine cancer and ezrin expression