Anat Cell Biol.
2010 Mar;43(1):54-63. 10.5115/acb.2010.43.1.54.
Effects of repeated restraint stress on platelet endothelial cell adhesion molecule-1 immunoreactivity and protein levels in the gerbil hippocampus after transient cerebral ischemia
- Affiliations
-
- 1Department of Anatomy and Neurobiology, and Institute of Neurodegeneration and Neuroregeneration, College of Medicine, Hallym University, Chuncheon, Korea. mhwon@hallym.ac.kr
- 2Department of Anatomy and Cell Biology, College of Veterinary Medicine and Research Institute for Veterinary Science, Seoul National University, Seoul, Korea.
- KMID: 2168897
- DOI: http://doi.org/10.5115/acb.2010.43.1.54
Abstract
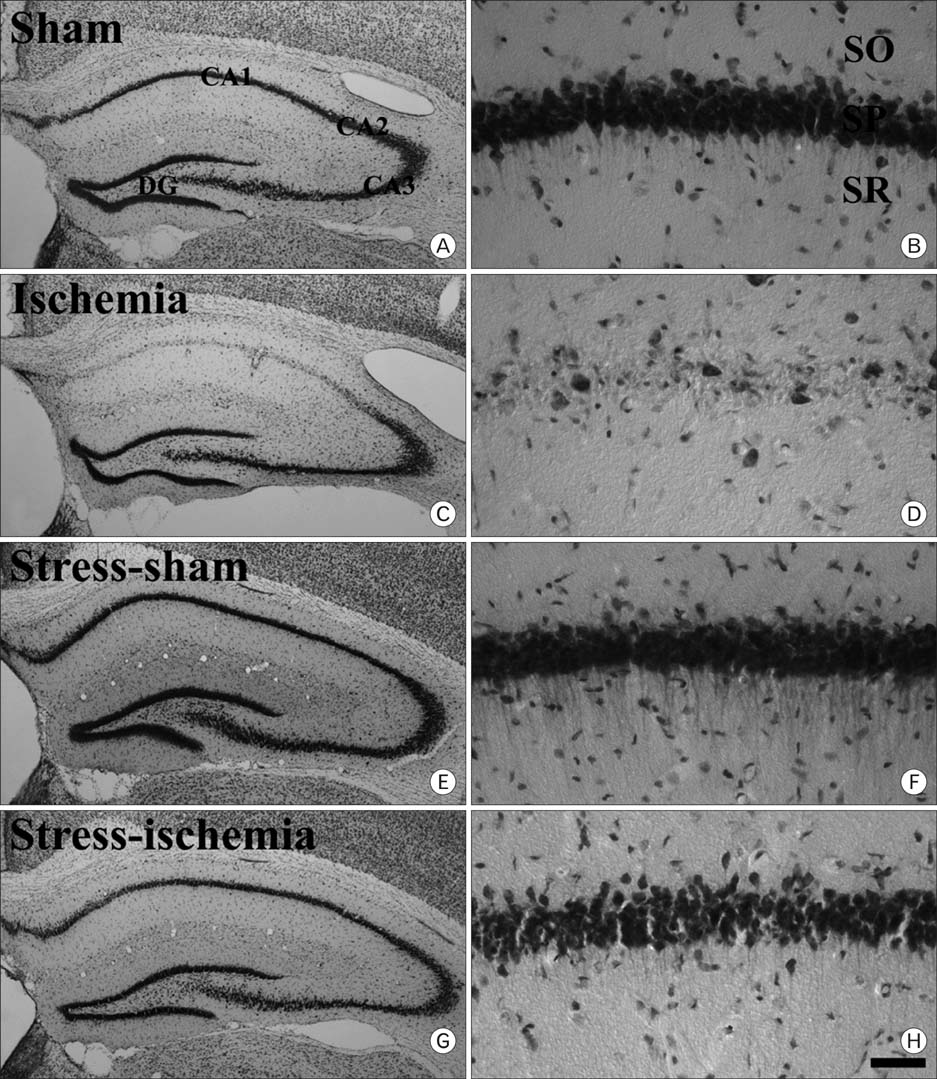
- Stress has long been known to be a causative factor of various disease states. In this study, we investigated the effects of repeated restraint stress on platelet endothelial cell adhesion molecule-1 (PECAM-1), a very important mediator in inflammation, immunoreactivity and protein levels as well as neuronal damage, in the gerbil hippocampus after 5 minutes of transient cerebral ischemia. Transient ischemia-induced neuronal death was shown in CA1 pyramidal cells 4 days after ischemia/reperfusion. However, repeated restraint stress protected neuronal death induced by ischemic damage. In the ischemia-group, PECAM-1 immunoreactivity and its protein levels were significantly increased in all the hippocampal subregions 4 days after ischemia/reperfusion. However, PECAM-1 immunoreactivity and its protein levels did not change significantly in the hippocampus of the stress-ischemia-group compared to the sham-groups. These results indicate that repeated restraint stress protects neuronal damage induced by transient cerebral ischemia, and this may be associated with maintenance of PECAM-1levels.
Keyword
MeSH Terms
Figure
Reference
-
1. Cancela LM, Bregonzio C, Molina VA. Anxiolytic-like effect induced by chronic stress is reversed by naloxone pretreatment. Brain Res Bull. 1995. 36:209–213.2. Dangerfield J, Larbi KY, Huang MT, Dewar A, Nourshargh S. PECAM-1 (CD31) homophilic interaction up-regulates alpha6beta1 on transmigrated neutrophils in vivo and plays a functional role in the ability of alpha6 integrins to mediate leukocyte migration through the perivascular basement membrane. J Exp Med. 2002. 196:1201–1211.3. Deak T, Bellamy C, D'Agostino LG. Exposure to forced swim stress does not alter central production of IL-1. Brain Res. 2003. 972:53–63.4. Deak T, Bordner KA, McElderry NK, et al. Stress-induced increases in hypothalamic IL-1: a systematic analysis of multiple stressor paradigms. Brain Res Bull. 2005. 64:541–556.5. Deng H, Han HS, Cheng D, Sun GH, Yenari MA. Mild hypothermia inhibits inflammation after experimental stroke and brain inflammation. Stroke. 2003. 34:2495–2501.6. DeVries A, Hung-Dong J, Bernard O, et al. Social stress exacerbates stroke outcome by suppressing Bcl-2 expression. Proc Natl Acad Sci USA. 2001. 98:11824–11828.7. Duncan GS, Andrew DP, Takimoto H, et al. Genetic evidence for functional redundancy of Platelet/Endothelial cell adhesion molecule-1 (PECAM-1): CD31-deficient mice reveal PECAM-1-dependent and PECAM-1-independent functions. J Immunol. 1999. 162:3022–3030.8. Fontella FU, Cimarosti H, Crema LM, et al. Acute and repeated restraint stress influences cellular damage in rat hippocampal slices exposed to oxygen and glucose deprivation. Brain Res Bull. 2005. 65:443–450.9. Gautier S, Ouk T, Petrault O, Caron J, Bordet R. Neutrophils contribute to intracerebral haemorrhages after treatment with recombinant tissue plasminogen activator following cerebral ischaemia. Br J Pharmacol. 2009. 156:673–679.10. Gidday JM, Gasche YG, Copin JC, et al. Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am J Physiol Heart Circ Physiol. 2005. 289:H558–H568.11. Graesser D, Solowiej A, Bruckner M, et al. Altered vascular permeability and early onset of experimental autoimmune encephalomyelitis in PECAM-1-deficient mice. J Clin Invest. 2002. 109:383–392.12. Gumina RJ, el Schultz J, Yao Z, et al. Antibody to platelet/endothelial cell adhesion molecule-1 reduces myocardial infarct size in a rat model of ischemia-reperfusion injury. Circulation. 1996. 94:3327–3333.13. Hendryk S, Czuba Z, Jedrzejewska-Szypułka H, Bazowski P, Dolezych H, Król W. Increase in activity of neutrophils and proinflammatory mediators in rats following acute and prolonged focal cerebral ischemia and reperfusion. Acta Neurochir Suppl. 2010. 106:29–35.14. Hwang IK, Kim DW, Yoo KY, et al. Ischemia-induced changes of platelet endothelial cell adhesion molecule-1 in the hippocampal CA1 region in gerbils. Brain Res. 2005. 1048:251–257.15. Iadecola C, Alexander M. Cerebral ischemia and inflammation. Curr Opin Neurol. 2001. 14:89–94.16. Ilan N, Madri JA. PECAM-1: old friend, new partners. Curr Opin Cell Biol. 2003. 15:515–524.17. Kalinowska A, Losy J. PECAM-1, a key player in neuroinflammation. Eur J Neurol. 2006. 13:1284–1290.18. Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982. 239:57–69.19. Lachuer J, Delton I, Buda M, Tappaz M. The habituation of brainstem catecholaminergic groups to chronic daily restraint stress is stress specific like that of the hypothalamo-pituitary-adrenal axis. Brain Res. 1994. 638:196–202.20. Loskota WA, Lomax P, Verity MA. A stereotaxic atlas of the Mongolian Gerbil Brain (Meriones unguiculatus). 1974. Ann Arbor: Ann Arbor Science Publishers Inc..21. Madrigal JL, Caso JR, de Cristóbal J, et al. Effect of subacute and chronic immobilisation stress on the outcome of permanent focal cerebral ischaemia in rats. Brain Res. 2003. 979:137–145.22. Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: comparison of stressors. Neuroscience. 1995. 69:83–88.23. Magariños AM, McEwen BS, Flügge G, Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci. 1996. 16:3534–3540.24. Maier SF, Nguyen KT, Deak T, Milligan ED, Watkins LR. Stress, learned helplessness, and brain interleukin-1 beta. Adv Exp Med Biol. 1999. 461:235–249.25. Martí O, Gavaldà A, Jolín T, Armario A. Effect of regularity of exposure to chronic immobilization stress on the circadian pattern of pituitary adrenal hormones, growth hormone, and thyroid stimulating hormone in the adult male rat. Psychoneuroendocrinology. 1993. 18:67–77.26. McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000. 886:172–189.27. Michiels C, Arnould T, Remacle J. Role of PECAM-1 in the adherence of PMN to hypoxic endothelial cells. Cell Adhes Commun. 1998. 5:367–374.28. Muller WA. The role of PECAM-1 (CD31) in leukocyte emigration: studies in vitro and in vivo. J Leukoc Biol. 1995. 57:523–528.29. Muller WA, Randolph GJ. Migration of leukocytes across endothelium and beyond: molecules involved in the transmigration and fate of monocytes. J Leukoc Biol. 1999. 66:698–704.30. Muller WA, Weigl SA, Deng X, Phillips DM. PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med. 1993. 178:449–460.31. O'Brien CD, Lim P, Sun J, Albelda SM. PECAM-1-dependent neutrophil transmigration is independent of monolayer PECAM-1 signaling or localization. Blood. 2003. 101:2816–2825.32. Petito CK, Pulsinelli WA. Sequential development of reversible and irreversible neuronal damage following cerebral ischemia. J Neuropathol Exp Neurol. 1984. 43:141–153.33. Rosenblum WI, Murata S, Nelson GH, Werner PK, Ranken R, Harmon RC. Anti-CD31 delays platelet adhesion/aggregation at sites of endothelial injury in mouse cerebral arterioles. Am J Pathol. 1994. 145:33–36.34. Schenkel AR, Chew TW, Muller WA. Platelet endothelial cell adhesion molecule deficiency or blockade significantly reduces leukocyte emigration in a majority of mouse strains. J Immunol. 2004. 173:6403–6408.35. Selye H. Stress and holistic medicine. Fam Community Health. 1980. 3:85–88.36. Solowiej A, Biswas P, Graesser D, Madri JA. Lack of platelet endothelial cell adhesion molecule-1 attenuates foreign body inflammation because of decreased angiogenesis. Am J Pathol. 2003. 162:953–962.37. Sugama S, Fujita M, Hashimoto M, Conti B. Stress induced morphological microglial activation in the rodent brain: involvement of interleukin-18. Neuroscience. 2007. 146:1388–1399.38. Sugo N, Hurn PD, Morahan B, Hattori K, Traystman RJ, DeVries AC. Social stress exacerbates focal cerebral ischemia in mice. Stroke. 2002. 33:1660–1664.39. Thompson RD, Noble KE, Larbi KY, et al. Platelet-endothelial cell adhesion molecule-1 (PECAM-1)-deficient mice demonstrate a transient and cytokine-specific role for PECAM-1 in leukocyte migration through the perivascular basement membrane. Blood. 2001. 97:1854–1860.40. Thoresen M, agenholm R, Løberg EM, Apriccna F. The stress of being restrained reduces brain damage after a hypoxic-ischaemic insult in the 7-day-old rat. Neuroreport. 1996. 7:481–484.41. Vaporciyan AA, DeLisser HM, Yan HC, et al. Involvement of platelet-endothelial cell adhesion molecule-1 in neutrophil recruitment in vivo. Science. 1993. 262:1580–1582.42. Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002. 22:6810–6818.43. Wang GJ, Deng HY, Maier CM, Sun GH, Yenari MA. Mild hypothermia reduces ICAM-1 expression, neutrophil infiltration and microglia/monocyte accumulation following experimental stroke. Neuroscience. 2002. 114:1081–1090.44. Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992. 588:341–345.45. Williams AJ, Berti R, Dave JR, Elliot PJ, Adams J, Tortella FC. Delayed treatment of ischemia/reperfusion brain injury: extended therapeutic window with the proteosome inhibitor MLN519. Stroke. 2004. 35:1186–1191.46. Zaremba J, Losy J. Adhesion molecules of immunoglobulin gene superfamily in stroke. Folia Morphol (Warsz). 2002a. 61:1–6.47. Zaremba J, Losy J. sPECAM-1 in serum and CSF of acute ischaemic stroke patients. Acta Neurol Scand. 2002b. 106:292–298.48. Zheng Z, Lee JE, Yenari MA. Stroke: molecular mechanisms and potential targets for treatment. Curr Mol Med. 2003. 3:361–372.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Change of peroxisome proliferator-activated receptor gamma expression pattern in the gerbil dentate gyrus after transient global cerebral ischemia
- The Effects of Repeated Restraint Stress on the Synaptic Plasticity in the Inner Molecular Layer of Mouse Dentate Gyrus
- The Effect of Delayed Administration of Green Tea Polyphenol, (-)-pigallocatechin-3-gallate, on the Change of Putrescine Level and Hippocampal Neuronal Cell Damage after Transient Global Ischemia in Gerbil
- Expression of Bcl-2 Protein in the Gerbil Hippocampus Following Transient Forebrain Ischemia and Its Modification by Ischemic Preconditioning
- Delayed Up-regulation of Vascular Endothelial Growth Factor and flk-1 after Global Cerebral Ischemia in Mongolian Gerbil: Possible roles in neuroangiogenesis?