Yonsei Med J.
2019 Oct;60(10):935-943. 10.3349/ymj.2019.60.10.935.
Distinct Neural Correlates of Executive Function by Amyloid Positivity and Associations with Clinical Progression in Mild Cognitive Impairment
- Affiliations
-
- 1Department of Psychiatry, College of Medicine, Chosun University, Gwangju, Korea.
- 2Premedical Science, College of Medicine, Chosun University, Gwangju, Korea. ehseo@chosun.ac.kr
- KMID: 2459146
- DOI: http://doi.org/10.3349/ymj.2019.60.10.935
Abstract
- PURPOSE
This study aimed to identify the neural basis of executive function (EF) in amnestic mild cognitive impairment (aMCI) according to beta-amyloid (Aβ) positivity. Furthermore, we explored if the identified brain areas could serve as predictors for clinical progression.
MATERIALS AND METHODS
We included individuals with aMCI using data from [¹â¸F]-florbetapir-positron emission tomography (PET), fluorodeoxyglucose-PET, and EF scores, as well as follow-up clinical severity scores at 1 and 5 years from baseline from the Alzheimer's Disease Neuroimaging Initiative database. The correlations between EF score and regional cerebral glucose metabolism (rCMglc) were analyzed separately for aMCI with low Aβ burden (aMCI Aβ−, n=230) and aMCI with high Aβ burden (aMCI Aβ+, n=268). Multiple linear regression analysis was conducted to investigate the associations between rCMglc and clinical progression.
RESULTS
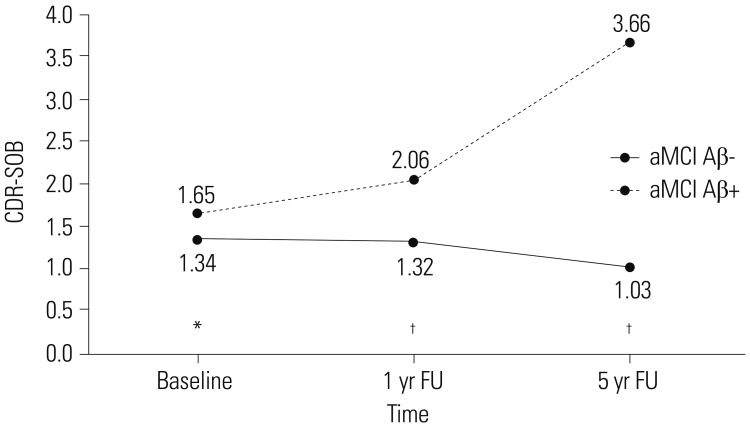
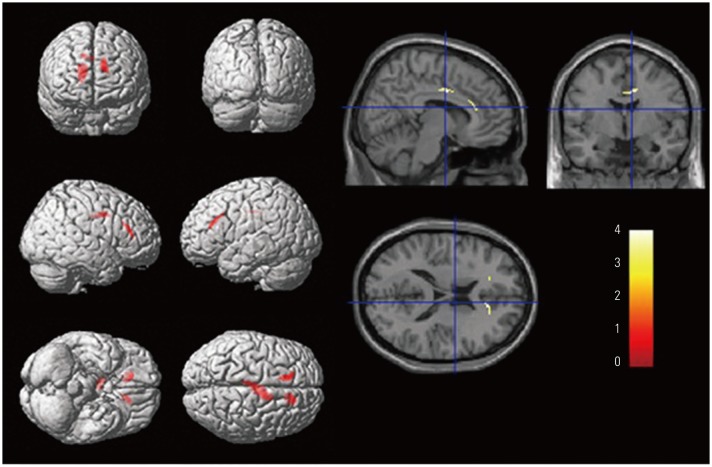
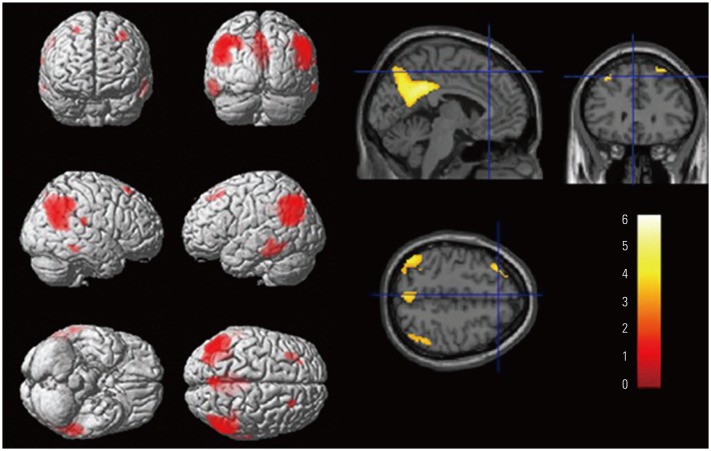
Longitudinal courses differed between aMCI Aβ− and aMCI Aβ+ groups. On average, aMCI Aβ− subjects maintained their level of clinical severity, whereas aMCI Aβ+ subjects showed progression. EF impairment in aMCI Aβ− was related to the anterior cingulate cortex (ACC), whereas that in aMCI Aβ+ was related to Alzheimer's Disease-vulnerable brain regions. ACC and the posterior cingulate cortex were associated with clinical progression in aMCI Aβ− and aMCI Aβ+, respectively.
CONCLUSION
Our findings suggest that although MCI subjects showed similar behavioral phenotypes at the time of diagnosis, EF and further progression were associated with different brain regions according to Aβ burden. Clarification of the etiologies and nature of EF impairment in aMCI are critical for disease prognosis and management.
Keyword
MeSH Terms
Figure
Reference
-
1. Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, et al. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004; 256:240–246. PMID: 15324367.2. Clark LR, Delano-Wood L, Libon DJ, McDonald CR, Nation DA, Bangen KJ, et al. Are empirically-derived subtypes of mild cognitive impairment consistent with conventional subtypes? J Int Neuropsychol Soc. 2013; 19:635–645. PMID: 23552486.
Article3. Nettiksimmons J, DeCarli C, Landau S, Beckett L. Alzheimer's Disease Neuroimaging Initiative. Biological heterogeneity in ADNI amnestic mild cognitive impairment. Alzheimers Dement. 2014; 10:511–521. PMID: 24418061.
Article4. Wolk DA, Price JC, Saxton JA, Snitz BE, James JA, Lopez OL, et al. Amyloid imaging in mild cognitive impairment subtypes. Ann Neurol. 2009; 65:557–568. PMID: 19475670.
Article5. Nordberg A, Carter SF, Rinne J, Drzezga A, Brooks DJ, Vandenberghe R, et al. A European multicentre PET study of fibrillar amyloid in Alzheimer's disease. Eur J Nucl Med Mol Imaging. 2013; 40:104–114. PMID: 22961445.
Article6. Ravaglia G, Forti P, Montesi F, Lucicesare A, Pisacane N, Rietti E, et al. Mild cognitive impairment: epidemiology and dementia risk in an elderly Italian population. J Am Geriatr Soc. 2008; 56:51–58. PMID: 18028343.
Article7. Pandya SY, Lacritz LH, Weiner MF, Deschner M, Woon FL. Predictors of reversion from mild cognitive impairment to normal cognition. Dement Geriatr Cogn Disord. 2017; 43:204–214. PMID: 28301848.
Article8. Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia--meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand. 2009; 119:252–265. PMID: 19236314.9. Brandt J, Aretouli E, Neijstrom E, Samek J, Manning K, Albert MS, et al. Selectivity of executive function deficits in mild cognitive impairment. Neuropsychology. 2009; 23:607–618. PMID: 19702414.
Article10. Kirova AM, Bays RB, Lagalwar S. Working memory and executive function decline across normal aging, mild cognitive impairment, and Alzheimer's disease. Biomed Res Int. 2015; 2015:748212. PMID: 26550575.
Article11. Seo EH, Kim H, Lee KH, Choo IH. Altered executive function in pre-mild cognitive impairment. J Alzheimers Dis. 2016; 54:933–940. PMID: 27567814.
Article12. Clement F, Gauthier S, Belleville S. Executive functions in mild cognitive impairment: emergence and breakdown of neural plasticity. Cortex. 2013; 49:1268–1279. PMID: 22841389.
Article14. Chang YL, Jacobson MW, Fennema-Notestine C, Hagler DJ Jr, Jennings RG, Dale AM, et al. Level of executive function influences verbal memory in amnestic mild cognitive impairment and predicts prefrontal and posterior cingulate thickness. Cereb Cortex. 2010; 20:1305–1313. PMID: 19776343.
Article15. Duara R, Loewenstein DA, Greig MT, Potter E, Barker W, Raj A, et al. Pre-MCI and MCI: neuropsychological, clinical, and imaging features and progression rates. Am J Geriatr Psychiatry. 2011; 19:951–960. PMID: 21422909.
Article16. Ewers M, Brendel M, Rizk-Jackson A, Rominger A, Bartenstein P, Schuff N, et al. Reduced FDG-PET brain metabolism and executive function predict clinical progression in elderly healthy subjects. Neuroimage Clin. 2014; 4:45–52. PMID: 24286024.
Article17. Wu L, Soder RB, Schoemaker D, Carbonnell F, Sziklas V, Rowley J, et al. Resting state executive control network adaptations in amnestic mild cognitive impairment. J Alzheimers Dis. 2014; 40:993–1004. PMID: 24583406.
Article18. Shon JM, Lee DY, Seo EH, Sohn BK, Kim JW, Park SY, et al. Functional neuroanatomical correlates of the executive clock drawing task (CLOX) performance in Alzheimer's disease: a FDG-PET study. Neuroscience. 2013; 246:271–280. PMID: 23673275.
Article19. Zheng D, Sun H, Dong X, Liu B, Xu Y, Chen S, et al. Executive dysfunction and gray matter atrophy in amnestic mild cognitive impairment. Neurobiol Aging. 2014; 35:548–555. PMID: 24119547.
Article20. Nho K, Risacher SL, Crane PK, DeCarli C, Glymour MM, Habeck C, et al. Voxel and surface-based topography of memory and executive deficits in mild cognitive impairment and Alzheimer's disease. Brain Imaging Behav. 2012; 6:551–567. PMID: 23070747.
Article21. Jueptner M, Weiller C. Review: does measurement of regional cerebral blood flow reflect synaptic activity? Implications for PET and fMRI. Neuroimage. 1995; 2:148–156. PMID: 9343597.22. Della Rosa PA, Cerami C, Gallivanone F, Prestia A, Caroli A, Castiglioni I, et al. A standardized [18F]-FDG-PET template for spatial normalization in statistical parametric mapping of dementia. Neuroinformatics. 2014; 12:575–593. PMID: 24952892.
Article23. Ma HR, Sheng LQ, Pan PL, Wang GD, Luo R, Shi HC, et al. Cerebral glucose metabolic prediction from amnestic mild cognitive impairment to Alzheimer's dementia: a meta-analysis. Transl Neurodegener. 2018; 7:9. PMID: 29713467.
Article24. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993; 43:2412–2414.25. Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, et al. Alzheimer's Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010; 74:201–209. PMID: 20042704.
Article26. Seo EH, Choo IL. Alzheimer's Disease Neuroimaging Initiative. Amyloid-independent functional neural correlates of episodic memory in amnestic mild cognitive impairment. Eur J Nucl Med Mol Imaging. 2016; 43:1088–1095. PMID: 26613793.
Article27. Gibbons LE, Carle AC, Mackin RS, Harvey D, Mukherjee S, Insel P, et al. A composite score for executive functioning, validated in Alzheimer's Disease Neuroimaging Initiative (ADNI) participants with baseline mild cognitive impairment. Brain Imaging Behav. 2012; 6:517–527. PMID: 22644789.
Article28. Landau SM, Mintun MA, Joshi AD, Koeppe RA, Petersen RC, Aisen PS, et al. Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann Neurol. 2012; 72:578–586. PMID: 23109153.
Article29. Dukart J, Mueller K, Horstmann A, Vogt B, Frisch S, Barthel H, et al. Differential effects of global and cerebellar normalization on detection and differentiation of dementia in FDG-PET studies. Neuroimage. 2010; 49:1490–1495. PMID: 19770055.
Article30. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002; 15:273–289. PMID: 11771995.
Article31. Ye BS, Kim HJ, Kim YJ, Jung NY, Lee JS, Lee J, et al. Longitudinal outcomes of amyloid positive versus negative amnestic mild cognitive impairments: a three-year longitudinal study. Sci Rep. 2018; 8:5557. PMID: 29615677.
Article32. Rowe CC, Bourgeat P, Ellis KA, Brown B, Lim YY, Mulligan R, et al. Predicting Alzheimer disease with β-amyloid imaging: results from the Australian imaging, biomarkers, and lifestyle study of ageing. Ann Neurol. 2013; 74:905–913. PMID: 24448836.
Article33. Mormino EC, Papp KV. Amyloid accumulation and cognitive decline in clinically normal older individuals: implications for aging and early Alzheimer's disease. J Alzheimers Dis. 2018; 64(s1):S633–S646. PMID: 29782318.
Article34. Pardo JV, Lee JT, Sheikh SA, Surerus-Johnson C, Shah H, Munch KR, et al. Where the brain grows old: decline in anterior cingulate and medial prefrontal function with normal aging. Neuroimage. 2007; 35:1231–1237. PMID: 17321756.
Article35. Heilbronner SR, Hayden BY. Dorsal anterior cingulate cortex: a bottom-up view. Annu Rev Neurosci. 2016; 39:149–170. PMID: 27090954.
Article36. Alexopoulos GS, Hoptman MJ, Kanellopoulos D, Murphy CF, Lim KO, Gunning FM. Functional connectivity in the cognitive control network and the default mode network in late-life depression. J Affect Disord. 2012; 139:56–65. PMID: 22425432.
Article37. Sheline YI, Raichle ME, Snyder AZ, Morris JC, Head D, Wang S, et al. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psychiatry. 2010; 67:584–587. PMID: 19833321.
Article38. Kim Y, Suh YL, Kim SJ, Bae MH, Kim JB, Kim Y, et al. The brain donation program in South Korea. Yonsei Med J. 2018; 59:1197–1204. PMID: 30450854.
Article39. Hamel R, Köhler S, Sistermans N, Koene T, Pijnenburg Y, van der Flier W, et al. The trajectory of cognitive decline in the pre-dementia phase in memory clinic visitors: findings from the 4C-MCI study. Psychol Med. 2015; 45:1509–1519. PMID: 25407094.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum to “Distinct Neural Correlates of ExecutiveFunction by Amyloid Positivity and Associations withClinical Progression in Mild Cognitive Impairment”by Yoon HJ, et al. (Yonsei Med J 2019;60(10):935-943.)
- Independent and Interactive Influences of the APOE Genotype and Beta-Amyloid Burden on Cognitive Function in Mild Cognitive Impairment
- Effects of Cognitive-based Interventions of Older Adults with Mild Cognitive Impairment: A Systematic Review and Meta-analysis
- The Effect of Clinical Characteristics and Subtypes on Amyloid Positivity in Patients with Amnestic Mild Cognitive Impairment
- The Effects of Exercise-Cognitive Combined Dual-Task Program on Cognitive Function and Depression in Elderly with Mild Cognitive Impairment