J Korean Med Sci.
2016 Feb;31(2):286-295. 10.3346/jkms.2016.31.2.286.
Independent and Interactive Influences of the APOE Genotype and Beta-Amyloid Burden on Cognitive Function in Mild Cognitive Impairment
- Affiliations
-
- 1Premedical Science, College of Medicine, Chosun University, Gwangju, Korea.
- 2Department of Neuropsychiatry, School of Medicine, Chosun University/Chosun University Hospital, Gwangju, Korea. ilhan.choo@chosun.ac.kr
- 3Department of Laboratory Medicine, School of Medicine, Chosun University/Chosun University Hospital, Gwangju, Korea.
- KMID: 2360055
- DOI: http://doi.org/10.3346/jkms.2016.31.2.286
Abstract
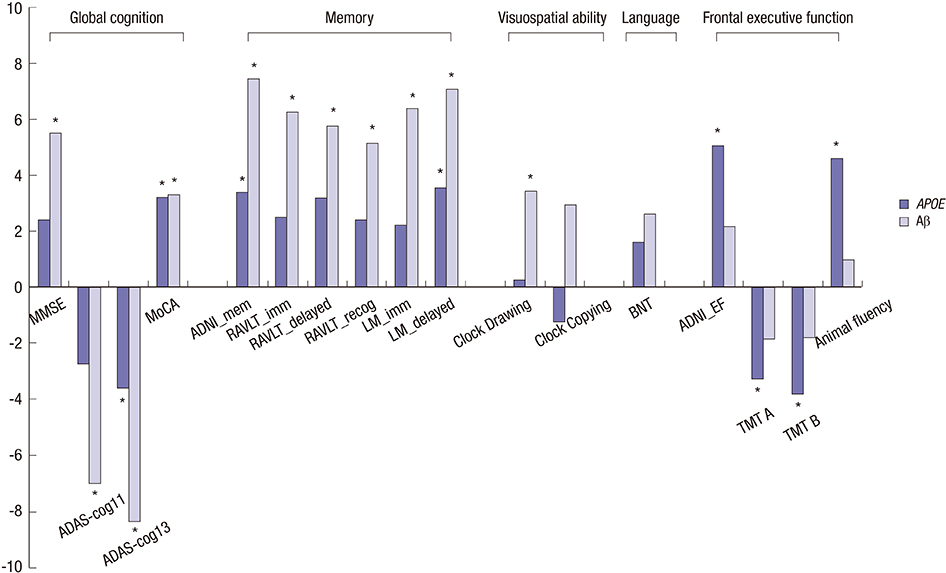
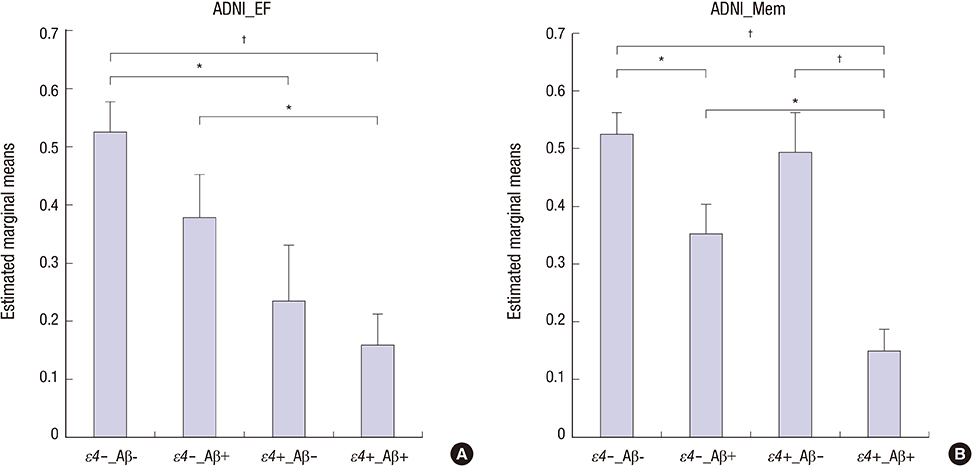
- This study aimed to investigate the independent and interactive influences of apolipoprotein E (APOE) epsilon4 and beta-amyloid (Abeta) on multiple cognitive domains in a large group of cognitively normal (CN) individuals and patients with mild cognitive impairment (MCI) and Alzheimer's disease (AD). Participants were included if clinical and cognitive assessments, amyloid imaging, and APOE genotype were all available from the Alzheimer's Disease Neuroimaging Initiative database (CN = 324, MCI = 502, AD = 182). Individuals with one or two copies of epsilon4 were designated as APOE epsilon4 carriers (epsilon4+); individuals with no epsilon4 were designated as APOE epsilon4 non-carriers (epsilon4-). Based on mean florbetapir standard uptake value ratios, participants were classified as Abeta burden-positive (Abeta+) or Abeta burden-negative (Abeta-). In MCI, APOE epsilon4 effects were predominantly observed on frontal executive function, with epsilon4+ participants exhibiting poorer performances; Abeta positivity had no influence on this effect. Abeta effects were observed on global cognition, memory, and visuospatial ability, with Abeta+ participants exhibiting poorer performances. Measures of frontal executive function were not influenced by Abeta. Interactive effects of APOE epsilon4+ and Abeta were observed on global cognition and verbal recognition memory. Abeta, not APOE epsilon4+, influenced clinical severity and functional status. The influences of APOE epsilon4+ and Abeta on cognitive function were minimal in CN and AD. In conclusion, we provide further evidence of both independent and interactive influences of APOE epsilon4+ and Abeta on cognitive function in MCI, with APOE epsilon4+ and Abeta showing dissociable effects on executive and non-executive functions, respectively.
Keyword
MeSH Terms
-
Aged
Aged, 80 and over
Alzheimer Disease/genetics/pathology
Amyloid beta-Peptides/*metabolism
Aniline Compounds/chemistry
Apolipoprotein E4/*genetics
Brain/radiography
Cognition
Databases, Factual
Demography
Ethylene Glycols/chemistry
Female
Genotype
Humans
Male
Mild Cognitive Impairment/genetics/*pathology
Positron-Emission Tomography
Amyloid beta-Peptides
Aniline Compounds
Apolipoprotein E4
Ethylene Glycols
Figure
Reference
-
1. Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, Berg L. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001; 58:397–405.2. Petersen RC, Caracciolo B, Brayne C, Gauthier S, Jelic V, Fratiglioni L. Mild cognitive impairment: a concept in evolution. J Intern Med. 2014; 275:214–228.3. Sohn BK, Yi D, Seo EH, Choe YM, Kim JW, Kim SG, Choi HJ, Byun MS, Jhoo JH, Woo JI, et al. Comparison of regional gray matter atrophy, white matter alteration, and glucose metabolism as a predictor of the conversion to Alzheimer’s disease in mild cognitive impairment. J Korean Med Sci. 2015; 30:779–787.4. Nordberg A, Carter SF, Rinne J, Drzezga A, Brooks DJ, Vandenberghe R, Perani D, Forsberg A, Långström B, Scheinin N, et al. A European multicentre PET study of fibrillar amyloid in Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2013; 40:104–114.5. Wolk DA, Price JC, Saxton JA, Snitz BE, James JA, Lopez OL, Aizenstein HJ, Cohen AD, Weissfeld LA, Mathis CA, et al. Amyloid imaging in mild cognitive impairment subtypes. Ann Neurol. 2009; 65:557–568.6. Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet. 2007; 39:17–23.7. Izaks GJ, Gansevoort RT, van der Knaap AM, Navis G, Dullaart RP, Slaets JP. The association of APOE genotype with cognitive function in persons aged 35 years or older. PLoS One. 2011; 6:e27415.8. Liu F, Pardo LM, Schuur M, Sanchez-Juan P, Isaacs A, Sleegers K, de Koning I, Zorkoltseva IV, Axenovich TI, Witteman JC, et al. The apolipoprotein E gene and its age-specific effects on cognitive function. Neurobiol Aging. 2010; 31:1831–1833.9. Kerchner GA, Berdnik D, Shen JC, Bernstein JD, Fenesy MC, Deutsch GK, Wyss-Coray T, Rutt BK. APOE epsilon4 worsens hippocampal CA1 apical neuropil atrophy and episodic memory. Neurology. 2014; 82:691–697.10. Wolk DA, Dickerson BC, Weiner M, Aiello M, Aisen P, Albert MS, Alexander G, Anderson HS, Anderson K, Apostolova L, et al. Apolipoprotein E (APOE) genotype has dissociable effects on memory and attentional-executive network function in Alzheimer’s disease. Proc Natl Acad Sci USA. 2010; 107:10256–10261.11. Chu CS, Lu T, Tsai SJ, Hong CJ, Yeh HL, Yang AC, Liu ME. APOE varepsilon4 polymorphism and cognitive deficit among the very old Chinese veteran men without dementia. Neurosci Lett. 2014; 576:17–21.12. Bretsky P, Guralnik JM, Launer L, Albert M, Seeman TE. MacArthur Studies of Successful Aging. The role of APOE-epsilon4 in longitudinal cognitive decline: MacArthur Studies of Successful Aging. Neurology. 2003; 60:1077–1081.13. Jack CR Jr, Lowe VJ, Weigand SD, Wiste HJ, Senjem ML, Knopman DS, Shiung MM, Gunter JL, Boeve BF, Kemp BJ, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: implications for sequence of pathological events in Alzheimer’s disease. Brain. 2009; 132:1355–1365.14. Landau SM, Mintun MA, Joshi AD, Koeppe RA, Petersen RC, Aisen PS, Weiner MW, Jagust WJ; Alzheimer’s Disease Neuroimaging Initiative. Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann Neurol. 2012; 72:578–586.15. Kantarci K, Lowe V, Przybelski SA, Weigand SD, Senjem ML, Ivnik RJ, Preboske GM, Roberts R, Geda YE, Boeve BF, et al. APOE modifies the association between Abeta load and cognition in cognitively normal older adults. Neurology. 2012; 78:232–240.16. Oh H, Mormino EC, Madison C, Hayenga A, Smiljic A, Jagust WJ. β-Amyloid affects frontal and posterior brain networks in normal aging. Neuroimage. 2011; 54:1887–1895.17. Rodrigue KM, Kennedy KM, Devous MD Sr, Rieck JR, Hebrank AC, Diaz-Arrastia R, Mathews D, Park DC. β-Amyloid burden in healthy aging: regional distribution and cognitive consequences. Neurology. 2012; 78:387–395.18. Dorey E, Chang N, Liu QY, Yang Z, Zhang W. Apolipoprotein E, amyloid-beta, and neuroinflammation in Alzheimer’s disease. Neurosci Bull. 2014; 30:317–330.19. Lim YY, Ellis KA, Pietrzak RH, Ames D, Darby D, Harrington K, Martins RN, Masters CL, Rowe C, Savage G, et al. Stronger effect of amyloid load than APOE genotype on cognitive decline in healthy older adults. Neurology. 2012; 79:1645–1652.20. Lim YY, Maruff P, Pietrzak RH, Ames D, Ellis KA, Harrington K, Lautenschlager NT, Szoeke C, Martins RN, Masters CL, et al. Effect of amyloid on memory and non-memory decline from preclinical to clinical Alzheimer’s disease. Brain. 2014; 137:221–231.21. Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, Jack CR Jr, Jagust WJ, Shaw LM, Toga AW, et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010; 74:201–209.22. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984; 34:939–944.23. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005; 53:695–699.24. Crane PK, Carle A, Gibbons LE, Insel P, Mackin RS, Gross A, Jones RN, Mukherjee S, Curtis SM, Harvey D, et al. Development and assessment of a composite score for memory in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Brain Imaging Behav. 2012; 6:502–516.25. Gibbons LE, Carle AC, Mackin RS, Harvey D, Mukherjee S, Insel P, Curtis SM, Mungas D, Crane PK; Alzheimer’s Disease Neuroimaging Initiative. A composite score for executive functioning, validated in Alzheimer’s Disease Neuroimaging Initiative (ADNI) participants with baseline mild cognitive impairment. Brain Imaging Behav. 2012; 6:517–527.26. Teng E, Becker BW, Woo E, Knopman DS, Cummings JL, Lu PH. Utility of the functional activities questionnaire for distinguishing mild cognitive impairment from very mild Alzheimer disease. Alzheimer Dis Assoc Disord. 2010; 24:348–353.27. Saykin AJ, Shen L, Foroud TM, Potkin SG, Swaminathan S, Kim S, Risacher SL, Nho K, Huentelman MJ, Craig DW, et al. Alzheimer’s Disease Neuroimaging Initiative biomarkers as quantitative phenotypes: genetics core aims, progress, and plans. Alzheimers Dement. 2010; 6:265–273.28. Liu Y, Yu JT, Wang HF, Han PR, Tan CC, Wang C, Meng XF, Risacher SL, Saykin AJ, Tan L. APOE genotype and neuroimaging markers of Alzheimer’s disease: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2015; 86:127–134.29. Wisdom NM, Callahan JL, Hawkins KA. The effects of apolipoprotein E on non-impaired cognitive functioning: a meta-analysis. Neurobiol Aging. 2011; 32:63–74.30. Fennema-Notestine C, Panizzon MS, Thompson WR, Chen CH, Eyler LT, Fischl B, Franz CE, Grant MD, Jak AJ, Jernigan TL, et al. Presence of ApoE epsilon4 allele associated with thinner frontal cortex in middle age. J Alzheimers Dis. 2011; 26:Suppl 3. 49–60.31. Hampel H. Amyloid-beta and cognition in aging and Alzheimer’s disease: molecular and neurophysiological mechanisms. J Alzheimers Dis. 2013; 33:Suppl 1. S79–86.32. Rodrigue KM, Kennedy KM, Park DC. Beta-amyloid deposition and the aging brain. Neuropsychol Rev. 2009; 19:436–450.33. Lim YY, Maruff P, Pietrzak RH, Ellis KA, Darby D, Ames D, Harrington K, Martins RN, Masters CL, Szoeke C, et al. Abeta and cognitive change: examining the preclinical and prodromal stages of Alzheimer’s disease. Alzheimers Dement. 2014; 10:743–751.e1.34. Knopman DS. beta-Amyloidosis and neurodegeneration in Alzheimer disease: who’s on first? Neurology. 2014; 82:1756–1757.35. Caselli RJ, Dueck AC, Osborne D, Sabbagh MN, Connor DJ, Ahern GL, Baxter LC, Rapcsak SZ, Shi J, Woodruff BK, et al. Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N Engl J Med. 2009; 361:255–263.36. Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW. Patterns of brain activation in people at risk for Alzheimer’s disease. N Engl J Med. 2000; 343:450–456.37. Hedden T, Oh H, Younger AP, Patel TA. Meta-analysis of amyloid-cognition relations in cognitively normal older adults. Neurology. 2013; 80:1341–1348.38. Jack CR Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010; 9:119–128.39. Chang YL, Fennema-Notestine C, Holland D, McEvoy LK, Stricker NH, Salmon DP, Dale AM, Bondi MW; Alzheimer’s Disease Neuroimaging Initiative. APOE interacts with age to modify rate of decline in cognitive and brain changes in Alzheimer’s disease. Alzheimers Dement. 2014; 10:336–348.40. Kleiman T, Zdanys K, Black B, Rightmer T, Grey M, Garman K, Macavoy M, Gelernter J, van Dyck C. Apolipoprotein E epsilon4 allele is unrelated to cognitive or functional decline in Alzheimer’s disease: retrospective and prospective analysis. Dement Geriatr Cogn Disord. 2006; 22:73–82.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Cognitive-based Interventions of Older Adults with Mild Cognitive Impairment: A Systematic Review and Meta-analysis
- Distinct Neural Correlates of Executive Function by Amyloid Positivity and Associations with Clinical Progression in Mild Cognitive Impairment
- The Effects of Exercise-Cognitive Combined Dual-Task Program on Cognitive Function and Depression in Elderly with Mild Cognitive Impairment
- Beta-amyloid imaging in dementia
- Correlation between Sleep and C-reactive Protein of Patients in Amnestic Mild Cognitive Impairment and Alzheimer’s Dementia