Nutr Res Pract.
2019 Jun;13(3):189-195. 10.4162/nrp.2019.13.3.189.
Anti-inflammatory effect of aged black garlic on 12-O-tetradecanoylphorbol-13-acetate-induced dermatitis in mice
- Affiliations
-
- 1Department of Food and Nutrition, Chungnam National University, 99 Daehak-ro, Yuseong-gu, Daejeon 34134, Korea. mrkim@cnu.ac.kr
- 2Korean Medicine-Application Center, Korea Institute of Oriental Medicine, Daegu 41062, Korea.
- KMID: 2453282
- DOI: http://doi.org/10.4162/nrp.2019.13.3.189
Abstract
- BACKGROUND/OBJECTIVES
Although aged black garlic has various biological activities such as anti-allergy, anti-inflammation and neuroprotection, effect of aged black garlic on chemically contact dermatitis is unclarified.
MATERIALS/METHODS
To evaluate anti-dermatitic activity of aged black garlic extract, we investigated effects of a fraction of aged black garlic extract (BG10) on both in vivo and in vitro.
RESULTS
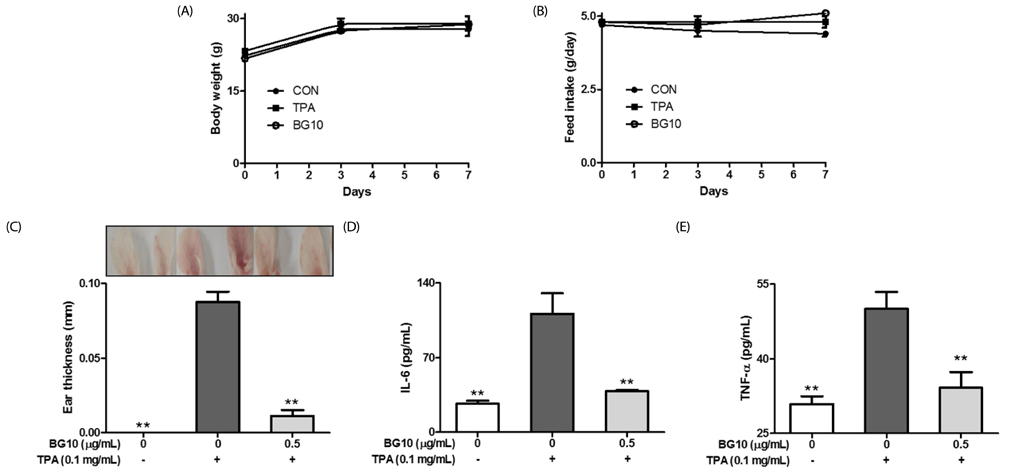
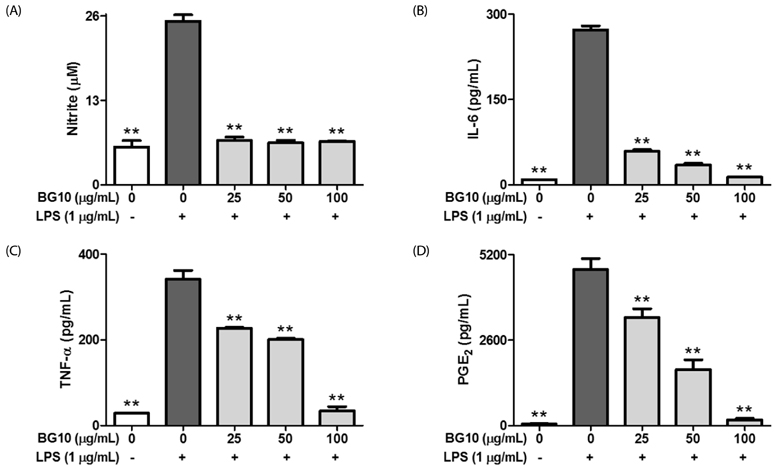
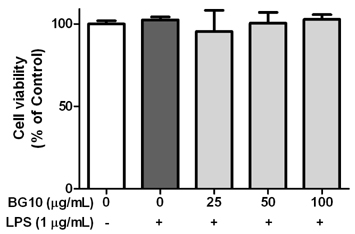
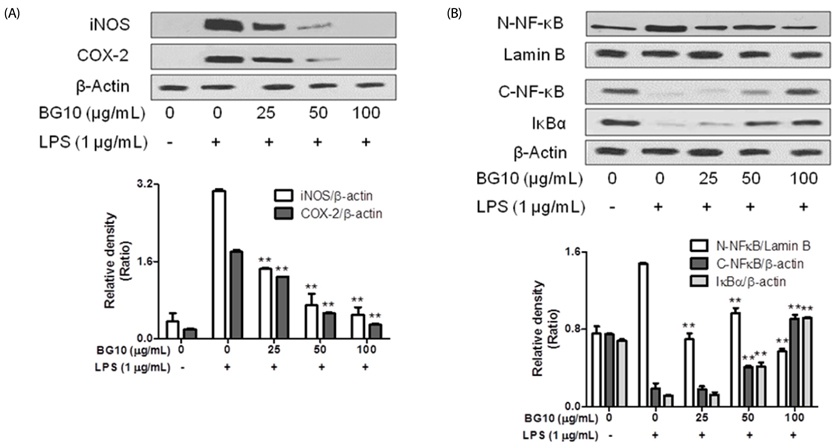
BG10 almost inhibited formation of nitric monoxide and interleukin-6 (IL-6; IC50, 7.07 µg/mL) at 25 µg/mL, and dose-dependently reduced production of tumor necrosis factor-α (TNF-α; IC50, 52.07 µg/mL) and prostaglandin E2 (IC50, 38.46 µg/mL) in lipopolysaccharide-stimulated RAW264.7 cells. In addition, BG10 significantly inhibited the expression of inducible nitric oxide synthase, cyclooxygenase-2 and nuclear NF-κB, and improved that of cytosolic levels of NF-κB and IκBα in the cells. Consistent with in vitro studies, BG10 (0.5 mg/mL) not only reduced ear edema but also suppressed the formation of IL-6 and TNF-α induced by 12-O-tetradecanoylphorbol-13-acetate in ear tissues of mice.
CONCLUSIONS
These findings suggest BG10 has anti-dermatitic activity through inhibiting activation of macrophages. Therefore, such effects of BG10 may provide information for the application of aged black garlic for prevention and therapy of contact dermatitis.
Keyword
MeSH Terms
-
Animals
Cyclooxygenase 2
Cytokines
Cytosol
Dermatitis*
Dermatitis, Contact
Dinoprostone
Ear
Edema
Garlic*
In Vitro Techniques
Inhibitory Concentration 50
Interleukin-6
Macrophages
Mice*
Necrosis
Neuroprotection
NF-kappa B
Nitric Oxide Synthase Type II
Cyclooxygenase 2
Cytokines
Dinoprostone
Interleukin-6
NF-kappa B
Nitric Oxide Synthase Type II
Figure
Reference
-
1. Dhingra N, Gulati N, Guttman-Yassky E. Mechanisms of contact sensitization offer insights into the role of barrier defects vs. intrinsic immune abnormalities as drivers of atopic dermatitis. J Invest Dermatol. 2013; 133:2311–2314.
Article2. Pérez-Pimiento AJ, Santaolalla M, de Paz S, Fernández-Parra B, Domínguez-Lázaro AR, Moneo I. Anaphylactic reaction to young garlic. Allergy. 1999; 54:626–629.
Article3. Itoh T, Ohguchi K, Iinuma M, Nozawa Y, Akao Y. Inhibitory effect of xanthones isolated from the pericarp of Garcinia mangostana L. on rat basophilic leukemia RBL-2H3 cell degranulation. Bioorg Med Chem. 2008; 16:4500–4508.
Article4. Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol. 2006; 6:218–230.
Article5. Butt MS, Sultan MT, Butt MS, Iqbal J. Garlic: nature's protection against physiological threats. Crit Rev Food Sci Nutr. 2009; 49:538–551.
Article6. Liu CT, Sheen LY, Lii CK. Does garlic have a role as an antidiabetic agent? Mol Nutr Food Res. 2007; 51:1353–1364.
Article7. Thomson M, Ali M. Garlic [Allium sativum]: a review of its potential use as an anti-cancer agent. Curr Cancer Drug Targets. 2003; 3:67–81.
Article8. Amagase H, Petesch BL, Matsuura H, Kasuga S, Itakura Y. Intake of garlic and its bioactive components. J Nutr. 2001; 131:955S–962S.
Article9. Lanzotti V. The analysis of onion and garlic. J Chromatogr A. 2006; 1112:3–22.
Article10. Borrelli F, Capasso R, Izzo AA. Garlic (Allium sativum L.): adverse effects and drug interactions in humans. Mol Nutr Food Res. 2007; 51:1386–1397.11. Burden AD, Wilkinson SM, Beck MH, Chalmers RJ. Garlic-induced systemic contact dermatitis. Contact Dermatitis. 1994; 30:299–300.
Article12. Moriguchi T, Saito H, Nishiyama N. Anti-ageing effect of aged garlic extract in the inbred brain atrophy mouse model. Clin Exp Pharmacol Physiol. 1997; 24:235–242.
Article13. Banerjee SK, Mukherjee PK, Maulik SK. Garlic as an antioxidant: the good, the bad and the ugly. Phytother Res. 2003; 17:97–106.
Article14. Bae SH, Lee SW, Kim MR, Kim JM, Suh HJ. Influence of steeping solution and storage temperature on the color change of garlic. J Food Sci. 2010; 75:C108–C112.
Article15. Imai J, Ide N, Nagae S, Moriguchi T, Matsuura H, Itakura Y. Antioxidant and radical scavenging effects of aged garlic extract and its constituents. Planta Med. 1994; 60:417–420.
Article16. Yoo JM, Sok DE, Kim MR. Anti-allergic action of aged black garlic extract in RBL-2H3 cells and passive cutaneous anaphylaxis reaction in mice. J Med Food. 2014; 17:92–102.
Article17. Kim MJ, Yoo YC, Kim HJ, Shin SK, Sohn EJ, Min AY, Sung NY, Kim MR. Aged black garlic exerts anti-inflammatory effects by decreasing no and proinflammatory cytokine production with less cytoxicity in LPS-stimulated raw 264.7 macrophages and LPS-induced septicemia mice. J Med Food. 2014; 17:1057–1063.
Article18. Nencini C, Menchiari A, Franchi GG, Micheli L. In vitro antioxidant activity of aged extracts of some Italian Allium species. Plant Foods Hum Nutr. 2011; 66:11–16.
Article19. Raschke WC, Baird S, Ralph P, Nakoinz I. Functional macrophage cell lines transformed by Abelson leukemia virus. Cell. 1978; 15:261–267.
Article20. Birasuren B, Kim NY, Jeon HL, Kim MR. Evaluation of the antioxidant capacity and phenolic content of agriophyllum pungens seed extracts from Mongolia. Prev Nutr Food Sci. 2013; 18:188–195.
Article21. Schwartz JN, Daniels CA, Shivers JC, Klintworth GK. Experimental cytomegalovirus ophthalmitis. Am J Pathol. 1974; 77:477–492.22. Fowler AJ, Sheu MY, Schmuth M, Kao J, Fluhr JW, Rhein L, Collins JL, Willson TM, Mangelsdorf DJ, Elias PM, Feingold KR. Liver X receptor activators display anti-inflammatory activity in irritant and allergic contact dermatitis models: liver-X-receptor-specific inhibition of inflammation and primary cytokine production. J Invest Dermatol. 2003; 120:246–255.
Article23. Jeon HL, Yoo JM, Lee BD, Lee SJ, Sohn EJ, Kim MR. Anti-inflammatory and antioxidant actions of N-arachidonoyl serotonin in RAW264.7 cells. Pharmacology. 2016; 97:195–206.
Article24. Hamidzadeh K, Christensen SM, Dalby E, Chandrasekaran P, Mosser DM. Macrophages and the recovery from acute and chronic inflammation. Annu Rev Physiol. 2017; 79:567–592.
Article25. Upadhyay S, Dixit M. Role of polyphenols and other phytochemicals on molecular signaling. Oxid Med Cell Longev. 2015; 2015:504253.
Article26. O'Neill LA. Toll-like receptor signal transduction and the tailoring of innate immunity: a role for Mal? Trends Immunol. 2002; 23:296–300.27. Seo SH, Jeong GS. Fisetin inhibits TNF-α-induced inflammatory action and hydrogen peroxide-induced oxidative damage in human keratinocyte HaCaT cells through PI3K/AKT/Nrf-2-mediated heme oxygenase-1 expression. Int Immunopharmacol. 2015; 29:246–253.
Article28. Bito T, Nishigori C. Impact of reactive oxygen species on keratinocyte signaling pathways. J Dermatol Sci. 2012; 68:3–8.
Article29. Park HJ, Jeon BT, Kim HC, Roh GS, Shin JH, Sung NJ, Han J, Kang D. Aged red garlic extract reduces lipopolysaccharide-induced nitric oxide production in RAW 264.7 macrophages and acute pulmonary inflammation through haeme oxygenase-1 induction. Acta Physiol (Oxf). 2012; 205:61–70.
Article30. Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016; 7:27–31.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antioxidant effect of garlic and aged black garlic in animal model of type 2 diabetes mellitus
- Two Cases of Irritant Contact Dermatitis due to Garlic
- A Case of Contact Dermatitis due to Garlic
- A Case of Irritant Contact Dermatitis due to Garlic
- 12-O-Tetradecanoylphorbol-13-Acetate Induces Keratin 8 Phosphorylation and Reorganization via Expression of Transglutaminase-2