Blood Res.
2019 Mar;54(1):31-37. 10.5045/br.2019.54.1.31.
Biochemical effects and safety of Gum arabic (Acacia Senegal) supplementation in patients with sickle cell anemia
- Affiliations
-
- 1Department of Physiology, Faculty of Medicine, Al Neelain University, Khartoum, Sudan. lamiskaddam@hotmail.com
- 2Department of Clinical Genetics, Faculty of Medicine, Al Neelain University, Khartoum, Sudan.
- 3Department of Hematology, Military Hospital Khartoum, Khartoum, Sudan.
- 4Department of Pediatrics, Military Hospital Khartoum, Khartoum, Sudan.
- 5Department of Community Medicine, Faculty of Medicine, Al Neelain University, Khartoum, Sudan.
- 6Department of Physiology, Faculty of Medicine, University of Khartoum, Khartoum, Sudan.
- KMID: 2451053
- DOI: http://doi.org/10.5045/br.2019.54.1.31
Abstract
- BACKGROUND
Sickle cell anemia (SCA) is a hereditary chronic hemolytic anemia with several clinical consequences. Intravascular sickling of red blood cells leads to multi-organ dysfunction. Moreover, several biochemical abnormalities have been associated with SCA. Gum arabic (GA) is an edible dried gummy exudate obtained from Acacia Senegal tree. GA showed antioxidant and cytoprotective activities and demonstrated protection against hepatic, renal, and cardiac toxicities in experimental rats. We hypothesized that regular intake of GA improves renal and liver functions in patients with SCA.
METHODS
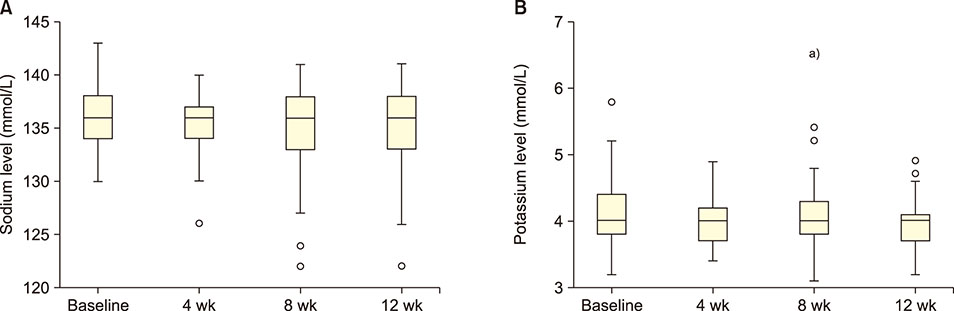
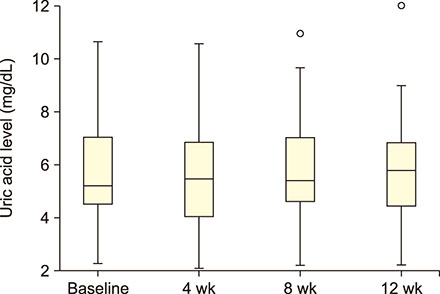
Forty-seven patients (5-42 yr) carrying hemoglobin SS were recruited. The patients received 30 g/day GA for 12 weeks. Blood samples were collected before administering GA and then after 4, 8, and 12 weeks. Liver enzymes, total protein, albumin, electrolytes, urea, creatinine, and uric acid were determined in the serum. The study was approved by the Al Neelain University Institutional Review Board and Research Ethics Committee Ministry of Health. The trial was registered at ClinicalTrials.gov (identifier: NCT02467257).
RESULTS
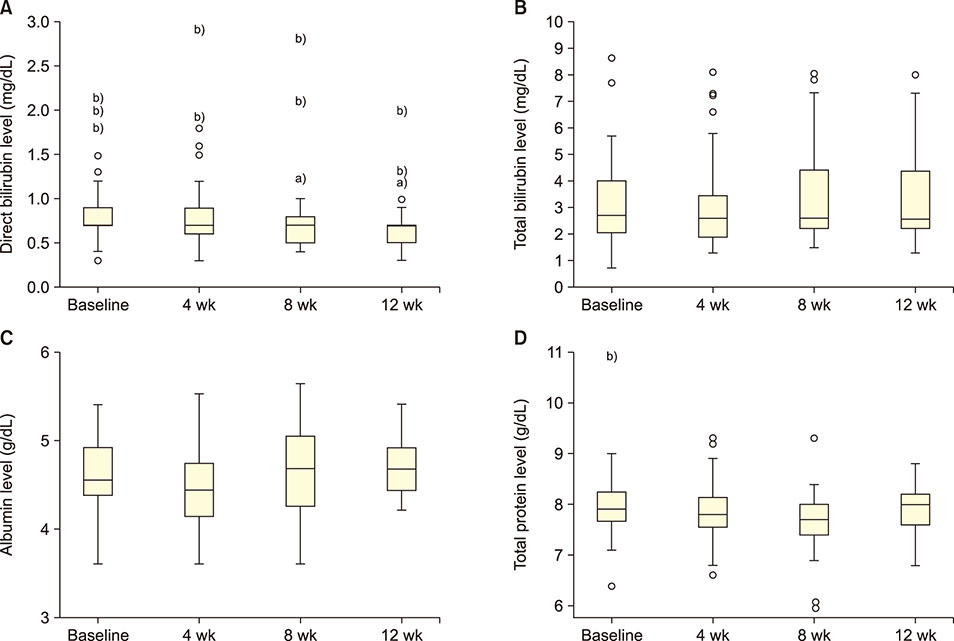
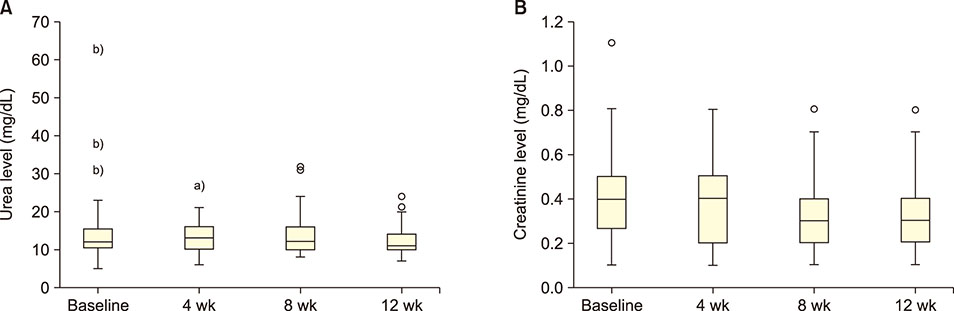
GA significantly decreased direct bilirubin level [statistical significance (P-value)=0.04]. It also significantly decreased serum alanine transaminase level after 4 weeks, which was sustained till the 8th week. GA, however, had no effect on serum aspartate transaminase level. In terms of renal function, GA decreased serum urea level but the effect was not sustained after the first month.
CONCLUSION
GA may alter the disease severity in SCA as demonstrated by its ability to decrease direct bilirubin and urea levels in the serum.
Keyword
MeSH Terms
-
Acacia
Alanine Transaminase
Anemia, Hemolytic
Anemia, Sickle Cell*
Animals
Aspartate Aminotransferases
Bilirubin
Cardiotoxicity
Creatinine
Electrolytes
Erythrocytes
Ethics Committees, Research
Exudates and Transudates
Gingiva*
Gum Arabic*
Hemoglobin, Sickle
Humans
Liver
Rats
Senegal
Trees
Urea
Uric Acid
Alanine Transaminase
Aspartate Aminotransferases
Bilirubin
Creatinine
Electrolytes
Gum Arabic
Hemoglobin, Sickle
Urea
Uric Acid
Figure
Reference
-
1. Modell B, Darlison M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull World Health Organ. 2008; 86:480–487.
Article2. Little JA, McGowan VR, Kato GJ, et al. Combination erythropoietin-hydroxyurea therapy in sickle cell disease: experience from the National Institutes of Health and a literature review. Haematologica. 2006; 91:1076–1083.3. Kaddam L, Fadl-Elmula I, Eisawi OA, et al. Gum Arabic as novel anti-oxidant agent in sickle cell anemia, phase II trial. BMC Hematol. 2017; 17:4.
Article4. Pandey S, Sharma A, Dahia S, et al. Biochemical indicator of sickle cell disease: preliminary report from India. Indian J Clin Biochem. 2012; 27:191–195.
Article5. Kotila T, Adedapo K, Adedapo A, Oluwasola O, Fakunle E, Brown B. Liver dysfunction in steady state sickle cell disease. Ann Hepatol. 2005; 4:261–263.
Article6. Nduka N, Kazem Y, Saleh B. Variation in serum electrolytes and enzyme concentrations in patients with sickle cell disease. J Clin Pathol. 1995; 48:648–651.
Article7. Yahaya IA. Biochemical features of hepatic dysfunction in Nigerians with sickle cell anaemia. Niger Postgrad Med J. 2012; 19:204–207.8. Oparinde DP, Oghagbon EK, Okesina AB, Olatunji PO, Ojuawo AO. Role of hepatic enzymes in the biochemical assessment of the severity of sickle cell anemia. Trop Gastroenterol. 2006; 27:118–121.9. Wright JG, Malia R, Cooper P, Thomas P, Preston FE, Serjeant GR. Protein C and protein S in homozygous sickle cell disease: does hepatic dysfunction contribute to low levels? Br J Haematol. 1997; 98:627–631.
Article10. Thompson J, Reid M, Hambleton I, Serjeant GR. Albuminuria and renal function in homozygous sickle cell disease: observations from a cohort study. Arch Intern Med. 2007; 167:701–708.
Article11. Aloni MN, Ngiyulu RM, Gini-Ehungu JL, et al. Renal function in children suffering from sickle cell disease: challenge of early detection in highly resource-scarce settings. PLoS One. 2014; 9:e96561.
Article12. Morgan AG, Serjeant GR. Renal function in patients over 40 with homozygous sickle-cell disease. Br Med J (Clin Res Ed). 1981; 282:1181–1183.
Article13. Gurkan S, Scarponi KJ, Hotchkiss H, Savage B, Drachtman R. Lactate dehydrogenase as a predictor of kidney involvement in patients with sickle cell anemia. Pediatr Nephrol. 2010; 25:2123–2127.
Article14. Agoreyo FO, Nwanze N. Plasma sodium and potassium changes in sickle cell patients. Int J Genet Mol Biol. 2010; 2:14–19.15. De Ceulaer K, Morgan AG, Choo-Kang E, Wilson WA, Serjeant GR. Serum urate concentrations in homozygous sickle cell disease. J Clin Pathol. 1981; 34:965–969.
Article16. Walker BR, Alexander F. Uric acid excretion in sickle cell anemia. JAMA. 1971; 215:255–258.
Article17. Rothschild BM, Sienknecht CW, Kaplan SB, Spindler JS. Sickle cell disease associated with uric acid deposition disease. Ann Rheum Dis. 1980; 39:392–395.
Article18. Morgan AG, De Ceulaer K, Serjeant GR. Glomerular function and hyperuricaemia in sickle cell disease. J Clin Pathol. 1984; 37:1046–1049.
Article19. Ali BH, Al-Husseni I, Beegam S, et al. Effect of gum arabic on oxidative stress and inflammation in adenine-induced chronic renal failure in rats. PLoS One. 2013; 8:e55242.
Article20. Al-Majed AA, Abd-Allah AR, Al-Rikabi AC, Al-Shabanah OA, Mostafa AM. Effect of oral administration of arabic gum on cisplatin-induced nephrotoxicity in rats. J Biochem Mol Toxicol. 2003; 17:146–153.
Article21. Kaddam L, FdleAlmula I, Eisawi OA, et al. Gum arabic as fetal hemoglobin inducing agent in sickle cell anemia; in vivo study. BMC Hematol. 2015; 15:19.
Article22. Palmer SM, Kaufman RA, Salamone SJ, et al. Cobas Integra: clinical laboratory instrument with continuous and random-access capabilities. Clin Chem. 1995; 41:1751–1760.
Article23. Gamal el-din AM, Mostafa AM, Al-Shabanah OA, Al-Bekairi AM, Nagi MN. Protective effect of arabic gum against acetaminophen-induced hepatotoxicity in mice. Pharmacol Res. 2003; 48:631–635.
Article24. Ali BH, Ziada A, Blunden G. Biological effects of gum arabic: a review of some recent research. Food Chem Toxicol. 2009; 47:1–8.
Article25. Ross AH, Eastwood MA, Brydon WG, Anderson JR, Anderson DM. A study of the effects of dietary gum arabic in humans. Am J Clin Nutr. 1983; 37:368–375.
Article26. Ali BH, Beegam S, Al-Lawati I, Waly MI, Al Za'abi M, Nemmar A. Comparative efficacy of three brands of gum acacia on adenine-induced chronic renal failure in rats. Physiol Res. 2013; 62:47–56.
Article27. Nasir O, Artunc F, Saeed A, et al. Effects of gum arabic (Acacia senegal) on water and electrolyte balance in healthy mice. J Ren Nutr. 2008; 18:230–238.
Article28. Khalid SA, Musa AM, Saeed AM, et al. Manipulating dietary fibre: Gum arabic making friends of the colon and the kidney. Bioactive carbohydr Diet Fibre. 2014; 3:71–76.
Article29. Al Mosawi AJ. The use of acacia gum in end stage renal failure. J Trop Pediatr. 2007; 53:362–365.
Article30. Nasir O, Umbach AT, Rexhepaj R, et al. Effects of gum arabic (Acacia senegal) on renal function in diabetic mice. Kidney Blood Press Res. 2012; 35:365–372.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Translocation Renal Cell Carcinoma t(6;11)(p21;q12) and Sickle Cell Anemia: First Report and Review of the Literature
- Genotypic influence of alpha-deletions on the phenotype of Indian sickle cell anemia patients
- Salmonella Typhi Osteomyelitis in a Non-sickle Cell Patient: Three Cases Report
- A Case of Sickle Cell Anemia with a Lack of High Frequency Red Blood Cell Antigen
- Promising role of voxelotor in managing sickle cell disease in children: a narrative review