Korean J Physiol Pharmacol.
2019 Jul;23(4):251-261. 10.4196/kjpp.2019.23.4.251.
Magnolol exerts anti-asthmatic effects by regulating Janus kinase-signal transduction and activation of transcription and Notch signaling pathways and modulating Th1/Th2/Th17 cytokines in ovalbumin-sensitized asthmatic mice
- Affiliations
-
- 1Department of Gerontology, Wujiang Hospital Affiliated to Nantong University, Suzhou, Jiangsu 215505, China. ISwoggerisits@yahoo.com
- KMID: 2450492
- DOI: http://doi.org/10.4196/kjpp.2019.23.4.251
Abstract
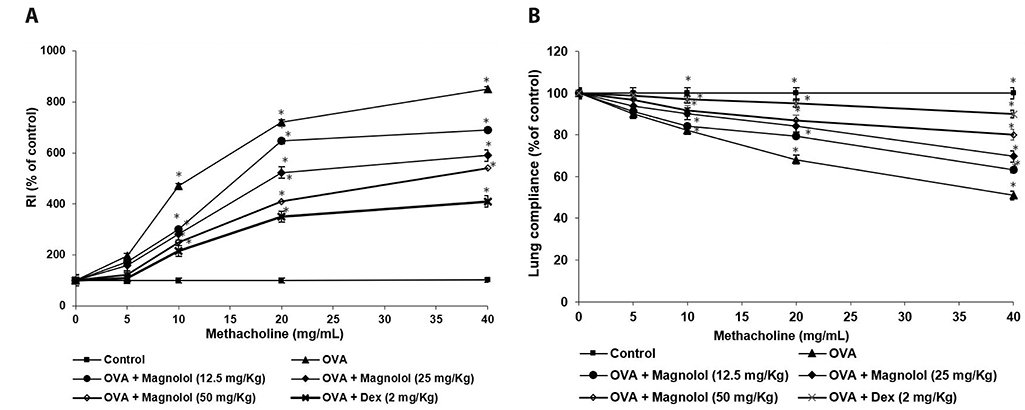
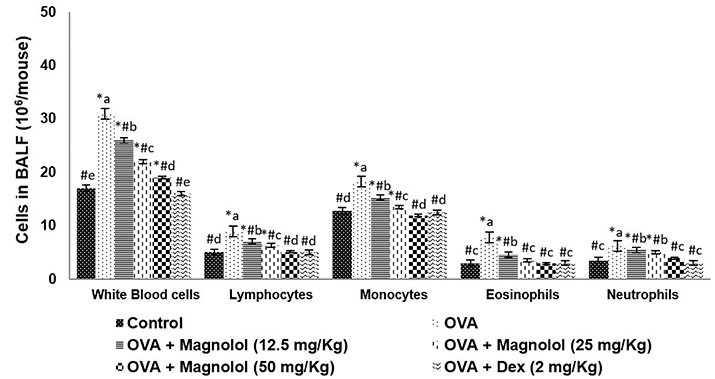
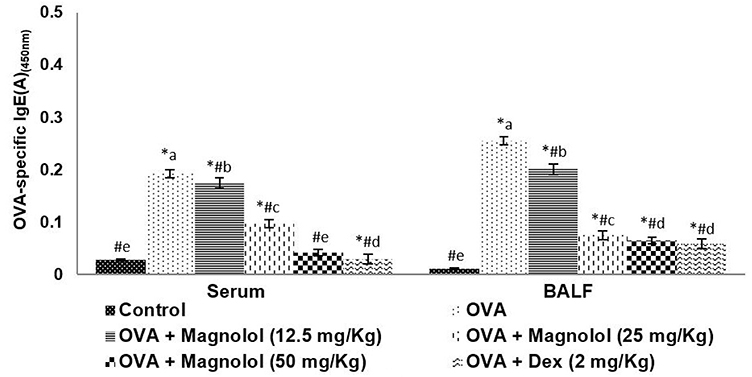
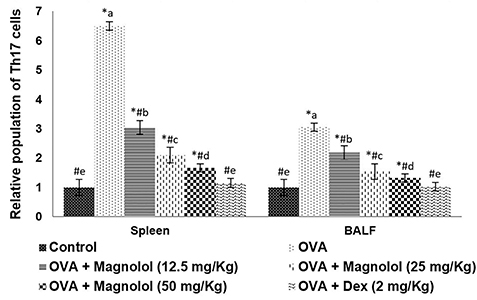
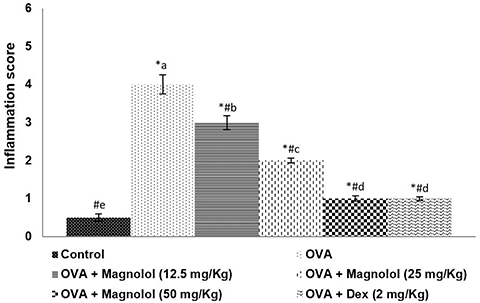
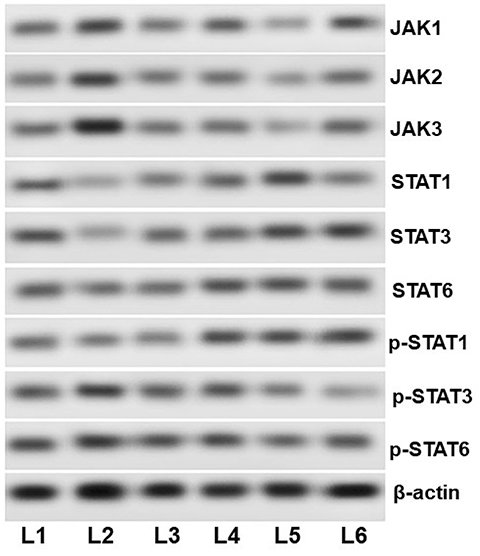
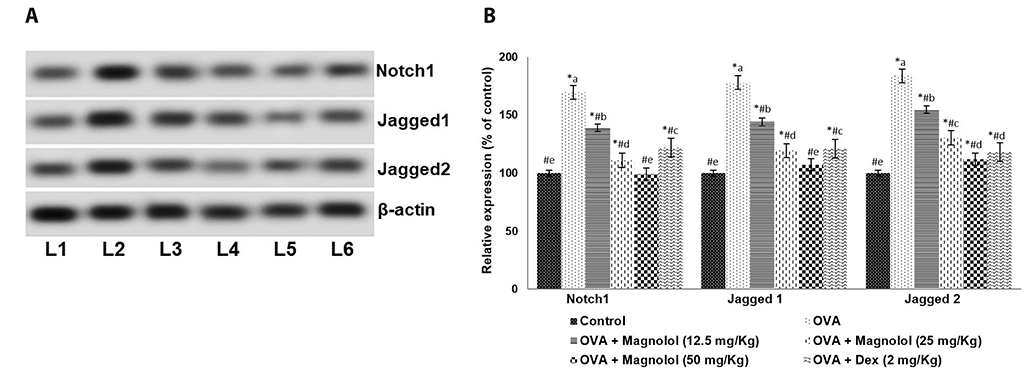
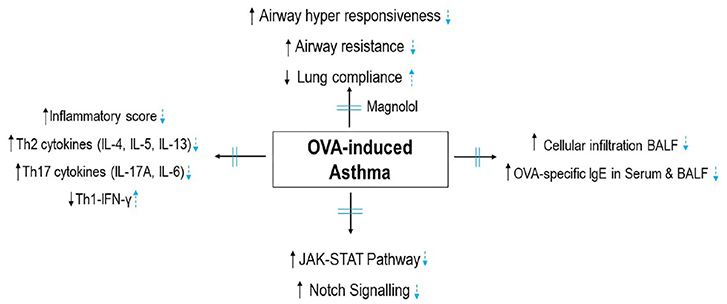
- Allergic asthma, is a common chronic inflammatory disease of the airway presenting with airway hyperresponsiveness and airway remodelling. T helper cells-derived cytokines are critically associated with asthma pathogenesis. Janus kinase-signal transduction and activation of transcription (JAK/STAT) signaling is found to be involved in asthma. Magnolol is a plant-derived bioactive compound with several pharmacological effects. The study aimed to assess the effects of magnolol in ovalbumin (OVA)-induced asthmatic model. BALB/c mice were sensitized and challenged with OVA. Magnolol (12.5, 25, or 50 mg/kg body weight) was administered to separate groups of animals. Dexamethasone was used as the positive control. Cellular infiltration into the bronchoalveolar lavage fluid (BALF) were reduced on magnolol treatment. The levels of Th2 and Th17 cytokines were reduced with noticeably raised levels of interferon gamma. Lung function was improved effectively along with restoration of bronchial tissue architecture. OVA-specific immunoglobulin E levels in serum and BALF were decreased by magnolol. Magnolol reduced Th17 cell population and effectively modulated the JAK-STAT and Notch 1 signaling. The results suggest the promising use of magnolol in therapy for allergic asthma.
Keyword
MeSH Terms
Figure
Reference
-
1. Pawankar R, Canonica GW, Holgate ST, Lockey RF, Blaiss M. WAO white book on allergy [Internet]. Milwaukee: World Allergy Organization;2013. cited 2017 Oct 16. Available from: http://www.worldallergy.org/UserFiles/file/ExecSummary-2013-v6-hires.pdf.2. Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008; 454:445–454.
Article3. Kim MS, Cho KA, Cho YJ, Woo SY. Effects of interleukin-9 blockade on chronic airway inflammation in murine asthma models. Allergy Asthma Immunol Res. 2013; 5:197–206.
Article4. Wegmann M. Th2 cells as targets for therapeutic intervention in allergic bronchial asthma. Expert Rev Mol Diagn. 2009; 9:85–100.
Article5. Bosnjak B, Stelzmueller B, Erb KJ, Epstein MM. Treatment of allergic asthma: modulation of Th2 cells and their responses. Respir Res. 2011; 12:114.
Article6. Simon D, Braathen LR, Simon HU. Eosinophils and atopic dermatitis. Allergy. 2004; 59:561–570.
Article7. Nakajima H, Hirose K. Role of IL-23 and Th17 cells in airway inflammation in asthma. Immune Netw. 2010; 10:1–4.
Article8. Brewer JM, Conacher M, Hunter CA, Mohrs M, Brombacher F, Alexander J. Aluminium hydroxide adjuvant initiates strong antigen-specific Th2 responses in the absence of IL-4- or IL-13-mediated signaling. J Immunol. 1999; 163:6448–6454.9. Hellings PW, Kasran A, Liu Z, Vandekerckhove P, Wuyts A, Overbergh L, Mathieu C, Ceuppens JL. Interleukin-17 orchestrates the granulocyte influx into airways after allergen inhalation in a mouse model of allergic asthma. Am J Respir Cell Mol Biol. 2003; 28:42–50.
Article10. Schmidt-Weber CB, Akdis M, Akdis CA. TH17 cells in the big picture of immunology. J Allergy Clin Immunol. 2007; 120:247–254.
Article11. Sergejeva S, Ivanov S, Lötvall J, Lindén A. Interleukin-17 as a recruitment and survival factor for airway macrophages in allergic airway inflammation. Am J Respir Cell Mol Biol. 2005; 33:248–253.
Article12. Ashino S, Takeda K, Li H, Taylor V, Joetham A, Pine PR, Gelfand EW. Janus kinase 1/3 signaling pathways are key initiators of TH2 differentiation and lung allergic responses. J Allergy Clin Immunol. 2014; 133:1162–1174.
Article13. Li RF, Wang GF. JAK/STAT5 signaling pathway inhibitor ruxolitinib reduces airway inflammation of neutrophilic asthma in mice model. Eur Rev Med Pharmacol Sci. 2018; 22:835–843.14. Banerjee S, Biehl A, Gadina M, Hasni S, Schwartz DM. JAK-STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs. 2017; 77:521–546.15. Hsieh YY, Chang CC, Hsu CM, Wan L, Chen SY, Lin WH, Tsai FJ. JAK-1 rs2780895 C-related genotype and allele but not JAK-1 rs10789166, rs4916008, rs2780885, rs17127114, and rs3806277 are associated with higher susceptibility to asthma. Genet Test Mol Biomarkers. 2011; 15:841–847.
Article16. Shen Y, Liu Y, Ke X, Kang HY, Hu GH, Hong SL. Association between JAK1 gene polymorphisms and susceptibility to allergic rhinitis. Asian Pac J Allergy Immunol. 2016; 34:124–129.
Article17. Gandhi NA, Bennett BL, Graham NM, Pirozzi G, Stahl N, Yancopoulos GD. Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov. 2016; 15:35–50.
Article18. Hu C, Li Z, Feng J, Tang Y, Qin L, Hu X, Zhang Y, He R. Glucocorticoids modulate Th1 and Th2 responses in asthmatic mouse models by inhibition of Notch1 signaling. Int Arch Allergy Immunol. 2018; 175:44–52.
Article19. Amsen D, Antov A, Flavell RA. The different faces of Notch in T-helper-cell differentiation. Nat Rev Immunol. 2009; 9:116–124.
Article20. Kang JH, Kim BS, Uhm TG, Lee SH, Lee GR, Park CS, Chung IY. Gamma-secretase inhibitor reduces allergic pulmonary inflammation by modulating Th1 and Th2 responses. Am J Respir Crit Care Med. 2009; 179:875–882.21. Guo XJ, Zhou M, Ren LP, Yang M, Huang SG, Xu WG. Small interfering RNA-mediated knockdown of Notch1 in lung. Chin Med J (Engl). 2009; 122:2647–2651.22. Vafeiadou K, Vauzour D, Lee HY, Rodriguez-Mateos A, Williams RJ, Spencer JP. The citrus flavanone naringenin inhibits inflammatory signalling in glial cells and protects against neuroinflammatory injury. Arch Biochem Biophys. 2009; 484:100–109.
Article23. Lim H, Park H, Kim HP. Effects of flavonoids on matrix metalloproteinase-13 expression of interleukin-1β-treated articular chondrocytes and their cellular mechanisms: inhibition of c-Fos/AP-1 and JAK/STAT signaling pathways. J Pharmacol Sci. 2011; 116:221–231.
Article24. Cheng YC, Tsao MJ, Chiu CY, Kan PC, Chen Y. Magnolol inhibits human glioblastoma cell migration by regulating N-cadherin. J Neuropathol Exp Neurol. 2018; 77:426–436.
Article25. Ranaware AM, Banik K, Deshpande V, Padmavathi G, Roy NK, Sethi G, Fan L, Kumar AP, Kunnumakkara AB. Magnolol: a neolignan from the magnolia family for the prevention and treatment of cancer. Int J Mol Sci. 2018; 19:2362.
Article26. Amorati R, Zotova J, Baschieri A, Valgimigli L. Antioxidant activity of magnolol and honokiol: kinetic and mechanistic investigations of their reaction with peroxyl radicals. J Org Chem. 2015; 80:10651–10659.
Article27. Huang SY, Tai SH, Chang CC, Tu YF, Chang CH, Lee EJ. Magnolol protects against ischemic-reperfusion brain damage following oxygen-glucose deprivation and transient focal cerebral ischemia. Int J Mol Med. 2018; 41:2252–2262.
Article28. National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the care and use of laboratory animals. 8th ed. Washington: National Academies Press;2011.29. Oh SW, Pae CI, Lee DK, Jones F, Chiang GK, Kim HO, Moon SH, Cao B, Ogbu C, Jeong KW, Kozu G, Nakanishi H, Kahn M, Chi EY, Henderson WR Jr. Tryptase inhibition blocks airway inflammation in a mouse asthma model. J Immunol. 2002; 168:1992–2000.
Article30. Djukanović R, Roche WR, Wilson JW, Beasley CR, Twentyman OP, Howarth RH, Holgate ST. Mucosal inflammation in asthma. Am Rev Respir Dis. 1990; 142:434–457.
Article31. Pichavant M, Goya S, Hamelmann E, Gelfand EW, Umetsu DT. Animal models of airway sensitization. Curr Protoc Immunol. 2007; Chapter 15:Unit 15.18.
Article32. Glaab T, Daser A, Braun A, Neuhaus-Steinmetz U, Fabel H, Alarie Y, Renz H. Tidal midexpiratory flow as a measure of airway hyperresponsiveness in allergic mice. Am J Physiol Lung Cell Mol Physiol. 2001; 280:L565–L573.
Article33. Duan W, Chan JH, Wong CH, Leung BP, Wong WS. Anti-inflammatory effects of mitogen-activated protein kinase kinase inhibitor U0126 in an asthma mouse model. J Immunol. 2004; 172:7053–7059.
Article34. Tabatabaian F, Ledford DK. Omalizumab for severe asthma: toward personalized treatment based on biomarker profile and clinical history. J Asthma Allergy. 2018; 11:53–61.
Article35. Holgate ST, Wenzel S, Postma DS, Weiss ST, Renz H, Sly PD. Asthma. Nat Rev Dis Primers. 2015; 1:15025.
Article36. Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015; 16:45–56.
Article37. Tritar-Cherif F, Ben M'Rad S, Merai S, Djenayah F. Corticotherapy for asthma in the child. Tunis Med. 2002; 80:1–6. French.38. Southworth T, Mason S, Bell A, Ramis I, Calbet M, Domenech A, Prats N, Miralpeix M, Singh D. PI3K, p38 and JAK/STAT signalling in bronchial tissue from patients with asthma following allergen challenge. Biomark Res. 2018; 6:14.
Article39. Kleiman A, Tuckermann JP. Glucocorticoid receptor action in beneficial and side effects of steroid therapy: lessons from conditional knockout mice. Mol Cell Endocrinol. 2007; 275:98–108.
Article40. Hocaoglu AB, Karaman O, Erge DO, Erbil G, Yilmaz O, Bagriyanik A, Uzuner N. Glycyrrhizin and long-term histopathologic changes in a murine model of asthma. Curr Ther Res Clin Exp. 2011; 72:250–261.
Article41. Oh SW, Cha JY, Jung JE, Chang BC, Kwon HJ, Lee BR, Kim DY. Curcumin attenuates allergic airway inflammation and hyper-responsiveness in mice through NF-κB inhibition. J Ethnopharmacol. 2011; 136:414–421.
Article42. Zhang W, Zhang X, Sheng A, Weng C, Zhu T, Zhao W, Li C. γ-Secretase inhibitor alleviates acute airway inflammation of allergic asthma in mice by downregulating Th17 cell differentiation. Mediators Inflamm. 2015; 2015:258168.
Article43. Deshmukh R, Kaundal M, Bansal V, Samardeep . Caffeic acid attenuates oxidative stress, learning and memory deficit in intra-cerebroventricular streptozotocin induced experimental dementia in rats. Biomed Pharmacother. 2016; 81:56–62.
Article44. Wenzel SE. Asthma: defining of the persistent adult phenotypes. Lancet. 2006; 368:804–813.
Article45. Turner DJ, Mulholland MW. Calcium signaling pathways in the enteric nervous system. Int J Surg Investig. 1999; 1:87–97.46. Elsner J, Kapp A. Regulation and modulation of eosinophil effector functions. Allergy. 1999; 54:15–26.
Article47. Wei M, Chu X, Guan M, Yang X, Xie X, Liu F, Chen C, Deng X. Protocatechuic acid suppresses ovalbumin-induced airway inflammation in a mouse allergic asthma model. Int Immunopharmacol. 2013; 15:780–788.
Article48. Lee MY, Seo CS, Lee JA, Lee NH, Kim JH, Ha H, Zheng MS, Son JK, Shin HK. Anti-asthmatic effects of Angelica dahurica against ovalbumin-induced airway inflammation via upregulation of heme oxygenase-1. Food Chem Toxicol. 2011; 49:829–837.
Article49. Busse WW, Lemanske RF Jr. Asthma. N Engl J Med. 2001; 344:350–362.
Article50. Busse WW, Coffman RL, Gelfand EW, Kay AB, Rosenwasser LJ. Mechanisms of persistent airway inflammation in asthma. A role for T cells and T-cell products. Am J Respir Crit Care Med. 1995; 152:388–393.
Article51. Cohn L, Tepper JS, Bottomly K. IL-4-independent induction of airway hyperresponsiveness by Th2, but not Th1, cells. J Immunol. 1998; 161:3813–3816.52. Bisset LR, Schmid-Grendelmeier P. Chemokines and their receptors in the pathogenesis of allergic asthma: progress and perspective. Curr Opin Pulm Med. 2005; 11:35–42.
Article53. Ngoc PL, Gold DR, Tzianabos AO, Weiss ST, Celedón JC. Cytokines, allergy, and asthma. Curr Opin Allergy Clin Immunol. 2005; 5:161–166.
Article54. Desai D, Brightling C. Cytokines and cytokine-specific therapy in asthma. Adv Clin Chem. 2012; 57:57–97.
Article55. Fish SC, Donaldson DD, Goldman SJ, Williams CM, Kasaian MT. IgE generation and mast cell effector function in mice deficient in IL-4 and IL-13. J Immunol. 2005; 174:7716–7724.
Article56. Kolls JK, Lindén A. Interleukin-17 family members and inflammation. Immunity. 2004; 21:467–476.
Article57. Wakashin H, Hirose K, Maezawa Y, Kagami S, Suto A, Watanabe N, Saito Y, Hatano M, Tokuhisa T, Iwakura Y, Puccetti P, Iwamoto I, Nakajima H. IL-23 and Th17 cells enhance Th2-cell-mediated eosinophilic airway inflammation in mice. Am J Respir Crit Care Med. 2008; 178:1023–1032.
Article58. Li J, Zhang B. Apigenin protects ovalbumin-induced asthma through the regulation of Th17 cells. Fitoterapia. 2013; 91:298–304.
Article59. Sun YC, Zhou QT, Yao WZ. Sputum interleukin-17 is increased and associated with airway neutrophilia in patients with severe asthma. Chin Med J (Engl). 2005; 118:953–956.60. Wilson RH, Whitehead GS, Nakano H, Free ME, Kolls JK, Cook DN. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. Am J Respir Crit Care Med. 2009; 180:720–730.
Article61. Ma C, Ma Z, Fu Q, Ma S. Curcumin attenuates allergic airway inflammation by regulation of CD4+CD25+ regulatory T cells (Tregs)/Th17 balance in ovalbumin-sensitized mice. Fitoterapia. 2013; 87:57–64.
Article62. Ji X, Han M, Yun Y, Li G, Sang N. Acute nitrogen dioxide (NO2) exposure enhances airway inflammation via modulating Th1/Th2 differentiation and activating JAK-STAT pathway. Chemosphere. 2015; 120:722–728.
Article63. Mao X, Ren Z, Parker GN, Sondermann H, Pastorello MA, Wang W, McMurray JS, Demeler B, Darnell JE Jr, Chen X. Structural bases of unphosphorylated STAT1 association and receptor binding. Mol Cell. 2005; 17:761–771.
Article64. O'Shea JJ, Kontzias A, Yamaoka K, Tanaka Y, Laurence A. Janus kinase inhibitors in autoimmune diseases. Ann Rheum Dis. 2013; 72:Suppl 2. ii111–ii115.65. O'Shea JJ, Holland SM, Staudt LM. JAKs and STATs in immunity, immunodeficiency, and cancer. N Engl J Med. 2013; 368:161–170.66. Zhong Z, Wen Z, Darnell JE Jr. Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994; 264:95–98.
Article67. Abroun S, Saki N, Ahmadvand M, Asghari F, Salari F, Rahim F. STATs: an old story, yet mesmerizing. Cell J. 2015; 17:395–411.68. Walford HH, Doherty TA. STAT6 and lung inflammation. JAKSTAT. 2013; 2:e25301.
Article69. Dallman MJ, Smith E, Benson RA, Lamb JR. Notch: control of lymphocyte differentiation in the periphery. Curr Opin Immunol. 2005; 17:259–266.
Article70. Osborne BA, Minter LM. Notch signalling during peripheral T-cell activation and differentiation. Nat Rev Immunol. 2007; 7:64–75.
Article71. Laky K, Fowlkes BJ. Notch signaling in CD4 and CD8 T cell development. Curr Opin Immunol. 2008; 20:197–202.
Article72. Zhang W, Nie Y, Chong L, Cai X, Zhang H, Lin B, Liang Y, Li C. PI3K and Notch signal pathways coordinately regulate the activation and proliferation of T lymphocytes in asthma. Life Sci. 2013; 92:890–895.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-inflammatory effects of Rhizopus spp. fermentation products on ovalbumin-induced asthma in mice
- Mega-dose vitamin C attenuated lung inflammation in mouse asthma model
- Immunomodulatory effects of ovalbumin extracts on splenocyte and macrophages in mice
- Genetic controls of Th17 cell differentiation and plasticity
- Regulation and Function of Th17 Cells