J Lipid Atheroscler.
2019 May;8(1):15-25. 10.12997/jla.2019.8.1.15.
Progression of Multifaceted Immune Cells in Atherosclerotic Development
- Affiliations
-
- 1Leading-edge Research Center for Drug Discovery and Development for Diabetes and Metabolic Disease, Kyungpook National University Hospital, Daegu, Korea. smpark93@gmail.com
- KMID: 2447753
- DOI: http://doi.org/10.12997/jla.2019.8.1.15
Abstract
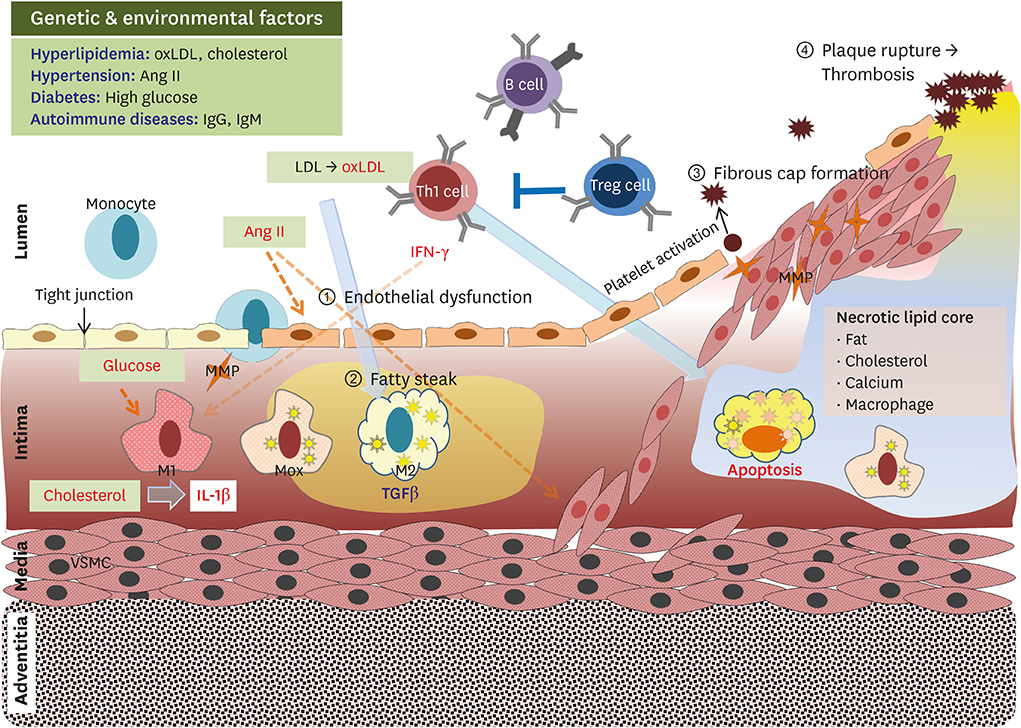
- Atherosclerosis is a major cause of morbidity and mortality due to cardiovascular diseases, such as coronary artery disease, stroke, and peripheral vascular disease, that are associated with thrombosis-induced organ infarction. In Westernized countries, the high prevalence of obesity-induced insulin resistance is predicted to be a major factor leading to atherosclerotic vascular disease. Both genetic and environmental factors interfere with immune responses in atherosclerosis development with chronic and non-resolving states. The most known autoimmune disease therapy is cytokine-targeted therapy, which targets tumor necrosis factor-α and interleukin (IL)-17 antagonists. Recently, a clinical trial with the anti-IL-1β antibody (canakinumab) had shown that the anti-inflammatory effects in canakinumab-treated subjects play a critical role in reducing cardiovascular disease prevalence. Recent emerging data have suggested effective therapeutics involving anti-obesity and anti-diabetic agents, as well as statin and anti-platelet drugs, for atherothrombosis prevention. It is well-known that specialized immune differentiation and activation completely depends on metabolic reprogramming mediated by mitochondrial dynamics in distinct immune cells. Therefore, there is a strong mechanistic link between metabolism and immune function mediated by mitochondrial function. In this review, we describe that cellular metabolism in immune cells is strongly interconnected with systemic metabolism in terms of diverse phenotypes and activation.
MeSH Terms
-
Atherosclerosis
Autoimmune Diseases
Autoimmunity
Cardiovascular Diseases
Coronary Artery Disease
Hydroxymethylglutaryl-CoA Reductase Inhibitors
Hypercholesterolemia
Infarction
Insulin Resistance
Interleukins
Metabolism
Mitochondrial Dynamics
Mortality
Necrosis
Peripheral Vascular Diseases
Phenotype
Prevalence
Stroke
Vascular Diseases
Interleukins
Figure
Reference
-
1. Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017; 38:2459–2472.
Article2. Proto JD, Doran AC, Subramanian M, Wang H, Zhang M, Sozen E, et al. Hypercholesterolemia induces T cell expansion in humanized immune mice. J Clin Invest. 2018; 128:2370–2375.
Article3. Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999; 340:115–126.4. Lusis AJ. Atherosclerosis. Nature. 2000; 407:233–241.
Article5. Daugherty A, Tall AR, Daemen MJAP, Falk E, Fisher EA, García-Cardeña G, et al. Recommendation on design, execution, and reporting of animal atherosclerosis studies: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol. 2017; 37:e131–e157.
Article6. Tabas I, Lichtman AH. Monocyte-macrophages and T cells in atherosclerosis. Immunity. 2017; 47:621–634.
Article7. Gisterå A, Hansson GK. The immunology of atherosclerosis. Nat Rev Nephrol. 2017; 13:368–380.
Article8. Feil S, Fehrenbacher B, Lukowski R, Essmann F, Schulze-Osthoff K, Schaller M, et al. Transdifferentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis. Circ Res. 2014; 115:662–667.
Article9. Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006; 444:860–867.
Article10. Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011; 12:204–212.
Article11. Fernandez-Ruiz I, Puchalska P, Narasimhulu CA, Sengupta B, Parthasarathy S. Differential lipid metabolism in monocytes and macrophages: influence of cholesterol loading. J Lipid Res. 2016; 57:574–586.
Article12. Yvan-Charvet L, Wang N, Tall AR. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler Thromb Vasc Biol. 2010; 30:139–143.
Article13. Wang R, Wang Y, Mu N, Lou X, Li W, Chen Y, et al. Activation of NLRP3 inflammasomes contributes to hyperhomocysteinemia-aggravated inflammation and atherosclerosis in apoE-deficient mice. Lab Invest. 2017; 97:922–934.
Article14. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017; 377:1119–1131.
Article15. Cochain C, Vafadarnejad E, Arampatzi P, Pelisek J, Winkels H, Ley K, et al. Single-cell RNA-seq reveals the transcriptional landscape and heterogeneity of aortic macrophages in murine atherosclerosis. Circ Res. 2018; 122:1661–1674.
Article16. Leentjens J, Bekkering S, Joosten LA, Netea MG, Burgner DP, Riksen NP. Trained innate immunity as a novel mechanism linking infection and the development of atherosclerosis. Circ Res. 2018; 122:664–669.
Article17. Chinetti-Gbaguidi G, Colin S, Staels B. Macrophage subsets in atherosclerosis. Nat Rev Cardiol. 2015; 12:10–17.
Article18. Kadl A, Meher AK, Sharma PR, Lee MY, Doran AC, Johnstone SR, et al. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ Res. 2010; 107:737–746.
Article19. Winkels H, Ehinger E, Vassallo M, Buscher K, Dinh HQ, Kobiyama K, et al. Atlas of the immune cell repertoire in mouse atherosclerosis defined by single-cell RNA-sequencing and mass cytometry. Circ Res. 2018; 122:1675–1688.
Article20. Lin JD, Nishi H, Poles J, Niu X, McCauley C, Rahman K, et al. Single-cell analysis of fate-mapped macrophages reveals heterogeneity, including stem-like properties, during atherosclerosis progression and regression. JCI Insight. 2019; 4:124574.
Article21. Kim K, Shim D, Lee JS, Zaitsev K, Williams JW, Kim KW, et al. Transcriptome analysis reveals nonfoamy rather than foamy plaque macrophages are proinflammatory in atherosclerotic murine models. Circ Res. 2018; 123:1127–1142.
Article22. Seijkens T, Hoeksema MA, Beckers L, Smeets E, Meiler S, Levels J, et al. Hypercholesterolemia-induced priming of hematopoietic stem and progenitor cells aggravates atherosclerosis. FASEB J. 2014; 28:2202–2213.
Article23. Bekkering S, Quintin J, Joosten LA, van der Meer JW, Netea MG, Riksen NP. Oxidized low-density lipoprotein induces long-term proinflammatory cytokine production and foam cell formation via epigenetic reprogramming of monocytes. Arterioscler Thromb Vasc Biol. 2014; 34:1731–1738.
Article24. Hoeksema MA, de Winther MP. Epigenetic regulation of monocyte and macrophage function. Antioxid Redox Signal. 2016; 25:758–774.
Article25. Fuster JJ, MacLauchlan S, Zuriaga MA, Polackal MN, Ostriker AC, Chakraborty R, et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science. 2017; 355:842–847.
Article26. van der Heijden T, Kritikou E, Venema W, van Duijn J, van Santbrink PJ, Slütter B, et al. Nlrp3 inflammasome inhibition by mcc950 reduces atherosclerotic lesion development in apolipoprotein e-deficient mice-brief report. Arterioscler Thromb Vasc Biol. 2017; 37:1457–1461.
Article27. Freemerman AJ, Johnson AR, Sacks GN, Milner JJ, Kirk EL, Troester MA, et al. Metabolic reprogramming of macrophages: glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype. J Biol Chem. 2014; 289:7884–7896.28. McGettrick AF, O'Neill LA. NLRP3 and IL-1β in macrophages as critical regulators of metabolic diseases. Diabetes Obes Metab. 2013; 15:Suppl 3. 19–25.
Article29. Van den Bossche J, O'Neill LA, Menon D. Macrophage immunometabolism: where are we (going)? Trends Immunol. 2017; 38:395–406.
Article30. Karunakaran D, Thrush AB, Nguyen MA, Richards L, Geoffrion M, Singaravelu R, et al. Macrophage mitochondrial energy status regulates cholesterol efflux and is enhanced by anti-mir33 in atherosclerosis. Circ Res. 2015; 117:266–278.
Article31. Baardman J, Verberk SGS, Prange KHM, van Weeghel M, van der Velden S, Ryan DG, et al. A defective pentose phosphate pathway reduces inflammatory macrophage responses during hypercholesterolemia. Cell Rep. 2018; 25:2044–2052.e5.
Article32. Hanna RN, Shaked I, Hubbeling HG, Punt JA, Wu R, Herrley E, et al. NR4A1 (Nur77) deletion polarizes macrophages toward an inflammatory phenotype and increases atherosclerosis. Circ Res. 2012; 110:416–427.
Article33. Bekkering S, van den Munckhof I, Nielen T, Lamfers E, Dinarello C, Rutten J, et al. Innate immune cell activation and epigenetic remodeling in symptomatic and asymptomatic atherosclerosis in humans in vivo. Atherosclerosis. 2016; 254:228–236.
Article34. Christ A, Günther P, Lauterbach MAR, Duewell P, Biswas D, Pelka K, et al. Western diet triggers NLRP3-dependent innate immune reprogramming. Cell. 2018; 172:162–175.e14.
Article35. Park S, Jeon JH, Min BK, Ha CM, Thoudam T, Park BY, et al. Role of the pyruvate dehydrogenase complex in metabolic remodeling: differential pyruvate dehydrogenase complex functions in metabolism. Diabetes Metab J. 2018; 42:270–281.
Article36. Libby P, Hansson GK. Taming immune and inflammatory responses to treat atherosclerosis. J Am Coll Cardiol. 2018; 71:173–176.
Article37. Eid RE, Rao DA, Zhou J, Lo SF, Ranjbaran H, Gallo A, et al. Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation. 2009; 119:1424–1432.
Article38. Burkett PR, Meyer zu Horste G, Kuchroo VK. Pouring fuel on the fire: Th17 cells, the environment, and autoimmunity. J Clin Invest. 2015; 125:2211–2219.
Article39. Zeboudj L, Maître M, Guyonnet L, Laurans L, Joffre J, Lemarie J, et al. Selective EGF-receptor inhibition in CD4+ T cells induces anergy and limits atherosclerosis. J Am Coll Cardiol. 2018; 71:160–172.
Article40. Simons KH, Aref Z, Peters HA, Welten SP, Nossent AY, Jukema JW, et al. The role of CD27-CD70-mediated T cell co-stimulation in vasculogenesis, arteriogenesis and angiogenesis. Int J Cardiol. 2018; 260:184–190.
Article41. Ryu H, Lim H, Choi G, Park YJ, Cho M, Na H, et al. Atherogenic dyslipidemia promotes autoimmune follicular helper T cell responses via IL-27. Nat Immunol. 2018; 19:583–593.
Article42. Westerterp M, Gautier EL, Ganda A, Molusky MM, Wang W, Fotakis P, et al. Cholesterol accumulation in dendritic cells links the inflammasome to acquired immunity. Cell Metab. 2017; 25:1294–1304.e6.
Article43. Cheng HY, Gaddis DE, Wu R, McSkimming C, Haynes LD, Taylor AM, et al. Loss of ABCG1 influences regulatory T cell differentiation and atherosclerosis. J Clin Invest. 2016; 126:3236–3246.
Article44. Tabas I, García-Cardeña G, Owens GK. Recent insights into the cellular biology of atherosclerosis. J Cell Biol. 2015; 209:13–22.
Article45. Rambold AS, Pearce EL. Mitochondrial dynamics at the interface of immune cell metabolism and function. Trends Immunol. 2018; 39:6–18.
Article46. Li J, McArdle S, Gholami A, Kimura T, Wolf D, Gerhardt T, et al. CCR5+T-bet+Foxp3+ effector CD4 T cells drive atherosclerosis. Circ Res. 2016; 118:1540–1552.
Article47. Chaudhari SM, Sluimer JC, Koch M, Theelen TL, Manthey HD, Busch M, et al. Deficiency of HIF1α in antigen-presenting cells aggravates atherosclerosis and type 1 T-helper cell responses in mice. Arterioscler Thromb Vasc Biol. 2015; 35:2316–2325.
Article48. Angelin A, Gil-de-Gómez L, Dahiya S, Jiao J, Guo L, Levine MH, et al. Foxp3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metab. 2017; 25:1282–1293.e7.
Article49. Mathis KW. An impaired neuroimmune pathway promotes the development of hypertension in systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol. 2015; 309:R1074–R1077.
Article50. Suciu CF, Prete M, Ruscitti P, Favoino E, Giacomelli R, Perosa F. Oxidized low density lipoproteins: the bridge between atherosclerosis and autoimmunity. Possible implications in accelerated atherosclerosis and for immune intervention in autoimmune rheumatic disorders. Autoimmun Rev. 2018; 17:366–375.
Article51. Clement M, Guedj K, Andreata F, Morvan M, Bey L, Khallou-Laschet J, et al. Control of the T follicular helper-germinal center B-cell axis by CD8+ regulatory T cells limits atherosclerosis and tertiary lymphoid organ development. Circulation. 2015; 131:560–570.
Article52. Holmqvist ME, Wedrén S, Jacobsson LT, Klareskog L, Nyberg F, Rantapää-Dahlqvist S, et al. Rapid increase in myocardial infarction risk following diagnosis of rheumatoid arthritis amongst patients diagnosed between 1995 and 2006. J Intern Med. 2010; 268:578–585.
Article53. Murphy AJ, Tall AR. Disordered haematopoiesis and athero-thrombosis. Eur Heart J. 2016; 37:1113–1121.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Association with inflammatory cells and apolipoproteins to the progression of atherosclerosis
- Current Understanding of Dendritic Cells in Atherosclerosis
- Involvement of Immune Cell Network in Aortic Valve Stenosis: Communication between Valvular Interstitial Cells and Immune Cells
- Prevalence of foam cells and helper-T cells in atherosclerotic plaques of Korean patients with carotid atheroma
- Roles of Vascular Smooth Muscle Cells in Atherosclerotic Calcification