Obstet Gynecol Sci.
2019 May;62(3):157-165. 10.5468/ogs.2019.62.3.157.
Different expression of GSK3β and pS9GSK3β depending on phenotype of cervical cancer: possible association of GSK3β with squamous cell carcinoma and pS9GSK3β with adenocarcinoma
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Nowon Eulji Medical Center, Eulji University, Seoul, Korea.
- 2Department of Pathology, Nowon Eulji Medical Center, Eulji University, Seoul, Korea. hojunglee@eulji.ac.kr
- KMID: 2445073
- DOI: http://doi.org/10.5468/ogs.2019.62.3.157
Abstract
OBJECTIVE
This study aimed to analyze the expression pattern of glycogen synthase kinase 3β (GSK3β) and its phosphorylated forms, GSK3β phosphorylated at Ser9 (pS9GSK3β), and GSK3β phosphorylated at Tyr216 (pY216GSK3β), in cervical squamous cell carcinoma (SCC) and adenocarcinoma (AC).
METHODS
We performed immunohistochemical staining for GSK3β, pS9GSK3β, and pY216GSK3β in 64 SCC and 20 AC cases and compared their expression patterns between the 2 tumor types.
RESULTS
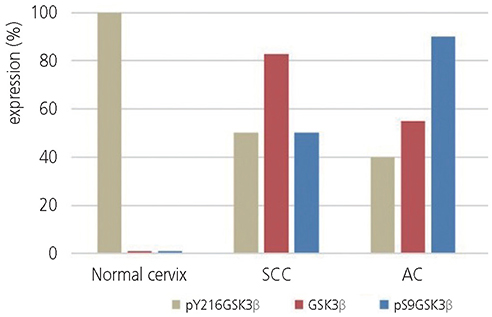
Increased GSK3β and pS9GSK3β expression but decreased pY216GSK3β expression compared with that in the normal cervix were observed in both SCC and AC specimens. Specifically, the levels of GSK3β and pS9GSK3β were significantly increased in SCC and AC, respectively. GSK3β was localized in the nucleus and/or cytoplasm of SCC and AC cells. However, pS9GSK3β was predominantly localized in the membrane of AC cells, whereas it was present in the nucleus and/or cytoplasm of SCC cells.
CONCLUSION
The results suggest that the phosphorylation status of GSK3β changes during cervical cancer development and the different expression levels and patterns of GSK3β and pS9GSK3β are associated with the specific histologic phenotype of cervical cancer.
Keyword
MeSH Terms
Figure
Reference
-
1. Wang SS, Sherman ME, Hildesheim A, Lacey JV Jr, Devesa S. Cervical adenocarcinoma and squamous cell carcinoma incidence trends among white women and black women in the United States for 1976–2000. Cancer. 2004; 100:1035–1044.
Article2. Green J, Berrington de Gonzalez A, Sweetland S, Beral V, Chilvers C, Crossley B, et al. Risk factors for adenocarcinoma and squamous cell carcinoma of the cervix in women aged 20–44 years: the UK National Case-Control Study of Cervical Cancer. Br J Cancer. 2003; 89:2078–2086.
Article3. Bertelsen BI, Steine SJ, Sandvei R, Molven A, Laerum OD. Molecular analysis of the PI3K-AKT pathway in uterine cervical neoplasia: frequent PIK3CA amplification and AKT phosphorylation. Int J Cancer. 2006; 118:1877–1883.4. Pett MR, Alazawi WO, Roberts I, Dowen S, Smith DI, Stanley MA, et al. Acquisition of high-level chromosomal instability is associated with integration of human papillomavirus type 16 in cervical keratinocytes. Cancer Res. 2004; 64:1359–1368.
Article5. Duensing S, Münger K. Centrosomes, genomic instability, and cervical carcinogenesis. Crit Rev Eukaryot Gene Expr. 2003; 13:9–23.
Article6. Manzo-Merino J, Contreras-Paredes A, Vázquez-Ulloa E, Rocha-Zavaleta L, Fuentes-Gonzalez AM, Lizano M. The role of signaling pathways in cervical cancer and molecular therapeutic targets. Arch Med Res. 2014; 45:525–539.
Article7. Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003; 116:1175–1186.
Article8. Mishra R. Glycogen synthase kinase 3 beta: can it be a target for oral cancer. Mol Cancer. 2010; 9:144.
Article9. Rath G, Jawanjal P, Salhan S, Nalliah M, Dhawan I. Clinical significance of inactivated glycogen synthase kinase 3β in HPV-associated cervical cancer: relationship with Wnt/β-catenin pathway activation. Am J Reprod Immunol. 2015; 73:460–478.
Article10. Shakoori A, Ougolkov A, Yu ZW, Zhang B, Modarressi MH, Billadeau DD, et al. Deregulated GSK3beta activity in colorectal cancer: its association with tumor cell survival and proliferation. Biochem Biophys Res Commun. 2005; 334:1365–1373.11. Zheng H, Saito H, Masuda S, Yang X, Takano Y. Phosphorylated GSK3beta-ser9 and EGFR are good prognostic factors for lung carcinomas. Anticancer Res. 2007; 27:3561–3569.12. Mishra R, Nagini S, Rana A. Expression and inactivation of glycogen synthase kinase 3 alpha/ beta and their association with the expression of cyclin D1 and p53 in oral squamous cell carcinoma progression. Mol Cancer. 2015; 14:20.
Article13. Park H, Lee M, Kim DW, Hong SY, Lee H. Glycogen synthase kinase 3β and cyclin D1 expression in cervical carcinogenesis. Obstet Gynecol Sci. 2016; 59:470–478.
Article14. Klaes R, Friedrich T, Spitkovsky D, Ridder R, Rudy W, Petry U, et al. Overexpression of p16(INK4A) as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int J Cancer. 2001; 92:276–284.
Article15. Lee H, Ro JY. Differential expression of GSK3β and pS9GSK3β in normal human tissues: can pS9GSK3β be an epithelial marker? Int J Clin Exp Pathol. 2015; 8:4064–4073.16. Gao C, Chen G, Kuan SF, Zhang DH, Schlaepfer DD, Hu J. FAK/PYK2 promotes the Wnt/β-catenin pathway and intestinal tumorigenesis by phosphorylating GSK3β. ELife. 2015; 4:e10072.
Article17. Darragh TM, Colgan TJ, Cox JT, Heller DS, Henry MR, Luff RD, et al. The lower anogenital squamous terminology standardization project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. J Low Genit Tract Dis. 2012; 16:205–242.18. Yim EK, Park JS. The role of HPV E6 and E7 oncoproteins in HPV-associated cervical carcinogenesis. Cancer Res Treat. 2005; 37:319–324.
Article19. Bello JO, Nieva LO, Paredes AC, Gonzalez AM, Zavaleta LR, Lizano M. Regulation of the Wnt/β-catenin signaling pathway by human papillomavirus E6 and E7 oncoproteins. Viruses. 2015; 7:4734–4755.
Article20. Zhang L, Wu J, Ling MT, Zhao L, Zhao KN. The role of the PI3K/Akt/mTOR signalling pathway in human cancers induced by infection with human papillomaviruses. Mol Cancer. 2015; 14:87.
Article21. Choi SK, Hong YO, Lee WM, Kim EK, Joo JE, Kim DW, et al. Overexpression of PI3K-p110α in the progression of uterine cervical neoplasia and its correlation with pAkt and DJ-1. Eur J Gynaecol Oncol. 2015; 36:389–393.22. Leis H, Segrelles C, Ruiz S, Santos M, Paramio JM. Expression, localization, and activity of glycogen synthase kinase 3beta during mouse skin tumorigenesis. Mol Carcinog. 2002; 35:180–185.23. Pérez-Plasencia C, Vázquez-Ortiz G, López-Romero R, Piña-Sanchez P, Moreno J, Salcedo M. Genome wide expression analysis in HPV16 cervical cancer: identification of altered metabolic pathways. Infect Agent Cancer. 2007; 2:16.
Article24. Ng SS, Mahmoudi T, Danenberg E, Bejaoui I, de Lau W, Korswagen HC, et al. Phosphatidylinositol 3-kinase signaling does not activate the wnt cascade. J Biol Chem. 2009; 284:35308–35313.
Article25. Naito S, Bilim V, Yuuki K, Ugolkov A, Motoyama T, Nagaoka A, et al. Glycogen synthase kinase-3beta: a prognostic marker and a potential therapeutic target in human bladder cancer. Clin Cancer Res. 2010; 16:5124–5132.26. Ougolkov AV, Fernandez-Zapico ME, Bilim VN, Smyrk TC, Chari ST, Billadeau DD. Aberrant nuclear accumulation of glycogen synthase kinase-3beta in human pancreatic cancer: association with kinase activity and tumor dedifferentiation. Clin Cancer Res. 2006; 12:5074–5081.27. Ougolkov AV, Fernandez-Zapico ME, Savoy DN, Urrutia RA, Billadeau DD. Glycogen synthase kinase-3beta participates in nuclear factor kappaB-mediated gene transcription and cell survival in pancreatic cancer cells. Cancer Res. 2005; 65:2076–2081.28. Beurel E, Jope RS. The paradoxical pro- and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog Neurobiol. 2006; 79:173–189.
Article29. Cole A, Frame S, Cohen P. Further evidence that the tyrosine phosphorylation of glycogen synthase kinase-3 (GSK3) in mammalian cells is an autophosphorylation event. Biochem J. 2004; 377:249–255.
Article30. Beurel E, Grieco SF, Jope RS. Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharmacol Ther. 2015; 148:114–131.
Article31. Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 2004; 29:95–102.
Article32. Zhou FQ, Zhou J, Dedhar S, Wu YH, Snider WD. NGF-induced axon growth is mediated by localized inactivation of GSK-3beta and functions of the microtubule plus end binding protein APC. Neuron. 2004; 42:897–912.33. Xu W, Ge Y, Liu Z, Gong R. Glycogen synthase kinase 3β orchestrates microtubule remodeling in compensatory glomerular adaptation to podocyte depletion. J Biol Chem. 2015; 290:1348–1363.
Article34. Debnath J, Walker SJ, Brugge JS. Akt activation disrupts mammary acinar architecture and enhances proliferation in an mTOR-dependent manner. J Cell Biol. 2003; 163:315–326.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Glycogen synthase kinase 3β and cyclin D1 expression in cervical carcinogenesis
- Effects of microRNA-135a on the epithelial–mesenchymal transition, migration and invasion of bladder cancer cells by targeting GSK3β through the Wnt/β-catenin signaling pathway
- β-carotene Inhibits Expression of c-Myc and Cyclin E in Helicobacter pylori-infected Gastric Epithelial Cells
- Two cases of synchronous squamous cell carcinoma and adenocarcinoma of the uterine cervix
- The Detection of the p53 Protein in Cervical Cancer and CIN by Immunohistochemistry