Dement Neurocogn Disord.
2018 Sep;17(3):110-119. 10.12779/dnd.2018.17.3.110.
¹â¸F-THK5351 PET Imaging in Nonfluent-Agrammatic Variant Primary Progressive Aphasia
- Affiliations
-
- 1Department of Neurology, Inha University School of Medicine, Incheon, Korea.
- 2Neuroscience Research Institute, Gachon University, Incheon, Korea.
- 3Department of Neuroscience, Gachon University College of Medicine, Incheon, Korea.
- 4Department of Neurology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 5Department of Neurology, Seoul Medical Center, Seoul, Korea.
- 6Department of Neurology, Gachon University Gil Medical Center, Incheon, Korea. ynoh@gachon.ac.kr
- 7Tohoku Medical and Pharmaceutical University, Sendai, Japan.
- 8Department of Neurology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
- 9Department of Health Science and Technology, GAIHST, Gachon University, Incheon, Korea.
- KMID: 2442800
- DOI: http://doi.org/10.12779/dnd.2018.17.3.110
Abstract
- BACKGROUND AND PURPOSE
To analyze 18F-THK5351 positron emission tomography (PET) scans of patients with clinically diagnosed nonfluent/agrammatic variant primary progressive aphasia (navPPA).
METHODS
Thirty-one participants, including those with Alzheimer's disease (AD, n=13), navPPA (n=3), and those with normal control (NC, n=15) who completed 3 Tesla magnetic resonance imaging, 18F-THK5351 PET scans, and detailed neuropsychological tests, were included. Voxel-based and region of interest (ROI)-based analyses were performed to evaluate retention of 18F-THK5351 in navPPA patients.
RESULTS
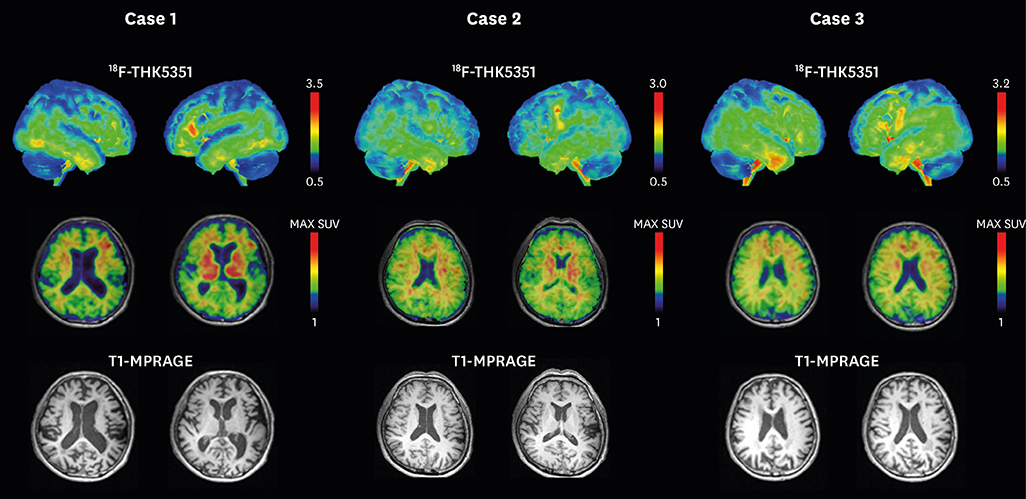
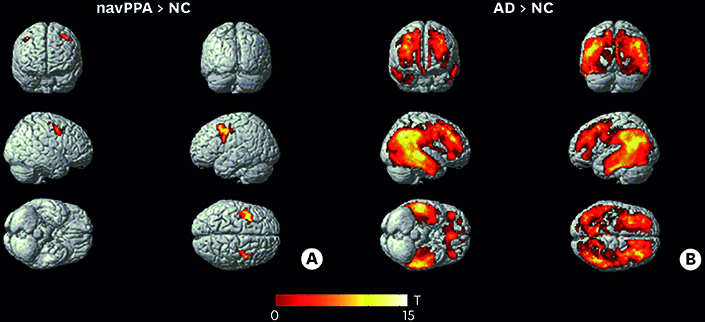
In ROI-based analysis, patients with navPPA had higher levels of THK retention in the Broca's area, bilateral inferior frontal lobes, bilateral precentral gyri, and bilateral basal ganglia. Patients with navPPA showed higher levels of THK retention in bilateral frontal lobes (mainly left side) compared than NC in voxel-wise analysis.
CONCLUSIONS
In our study, THK retention in navPPA patients was mainly distributed at the frontal region which was well correlated with functional-radiological distribution of navPPA. Our results suggest that tau PET imaging could be a supportive tool for diagnosis of navPPA in combination with a clinical history.
Keyword
MeSH Terms
Figure
Reference
-
1. Mesulam MM. Primary progressive aphasia--a language-based dementia. N Engl J Med. 2003; 349:1535–1542.
Article2. Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004; 55:335–346.
Article3. Josephs KA, Duffy JR, Strand EA, Whitwell JL, Layton KF, Parisi JE, et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006; 129:1385–1398.
Article4. Hodges JR, Davies RR, Xuereb JH, Casey B, Broe M, Bak TH, et al. Clinicopathological correlates in frontotemporal dementia. Ann Neurol. 2004; 56:399–406.
Article5. Kertesz A, McMonagle P, Blair M, Davidson W, Munoz DG. The evolution and pathology of frontotemporal dementia. Brain. 2005; 128:1996–2005.
Article6. Forman MS, Farmer J, Johnson JK, Clark CM, Arnold SE, Coslett HB, et al. Frontotemporal dementia: clinicopathological correlations. Ann Neurol. 2006; 59:952–962.
Article7. Knibb JA, Xuereb JH, Patterson K, Hodges JR. Clinical and pathological characterization of progressive aphasia. Ann Neurol. 2006; 59:156–165.
Article8. Harris JM, Gall C, Thompson JC, Richardson AM, Neary D, du Plessis D, et al. Classification and pathology of primary progressive aphasia. Neurology. 2013; 81:1832–1839.
Article9. Grossman M. Primary progressive aphasia: clinicopathological correlations. Nat Rev Neurol. 2010; 6:88–97.
Article10. Villemagne VL, Fodero-Tavoletti MT, Masters CL, Rowe CC. Tau imaging: early progress and future directions. Lancet Neurol. 2015; 14:114–124.
Article11. Harada R, Okamura N, Furumoto S, Furukawa K, Ishiki A, Tomita N, et al. 18F-THK5351: a novel PET radiotracer for imaging neurofibrillary pathology in Alzheimer disease. J Nucl Med. 2016; 57:208–214.
Article12. Harada R, Ishiki A, Kai H, Sato N, Furukawa K, Furumoto S, et al. Correlations of 18F-THK5351 PET with postmortem burden of tau and astrogliosis in Alzheimer disease. J Nucl Med. 2018; 59:671–674.
Article13. Ng KP, Pascoal TA, Mathotaarachchi S, Therriault J, Kang MS, Shin M, et al. Monoamine oxidase B inhibitor, selegiline, reduces 18F-THK5351 uptake in the human brain. Alzheimers Res Ther. 2017; 9:25.
Article14. Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011; 76:1006–1014.
Article15. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer's disease. Neurology. 1984; 34:939–944.
Article16. Kang Y, Jahng S, Na DL. Seoul Neuropsychological Screening Battery. 2nd ed. Seoul: Human Brain Research & Consulting Co.;2012.17. Borroni B, Agosti C, Premi E, Cerini C, Cosseddu M, Paghera B, et al. The FTLD-modified Clinical Dementia Rating scale is a reliable tool for defining disease severity in frontotemporal lobar degeneration: evidence from a brain SPECT study. Eur J Neurol. 2010; 17:703–707.
Article18. Kim EJ, Park KW, Lee JH, Choi S, Jeong JH, Yoon SJ, et al. Clinical and neuropsychological characteristics of a nationwide hospital-based registry of frontotemporal dementia patients in Korea: a CREDOS-FTD study. Dement Geriatr Cogn Dis Extra. 2014; 4:242–251.
Article19. Knopman DS, Kramer JH, Boeve BF, Caselli RJ, Graff-Radford NR, Mendez MF, et al. Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain. 2008; 131:2957–2968.
Article20. Lee JH, Kim SH, Kim GH, Seo SW, Park HK, Oh SJ, et al. Identification of pure subcortical vascular dementia using 11C-Pittsburgh compound B. Neurology. 2011; 77:18–25.
Article21. Ahn HJ, Chin J, Park A, Lee BH, Suh MK, Seo SW, et al. Seoul neuropsychological screening battery-dementia version (SNSB-D): a useful tool for assessing and monitoring cognitive impairments in dementia patients. J Korean Med Sci. 2010; 25:1071–1076.
Article22. Kim H, Na DL. Normative data on the Korean version of the Western Aphasia Battery. J Clin Exp Neuropsychol. 2004; 26:1011–1020.
Article23. Thurfjell L, Lilja J, Lundqvist R, Buckley C, Smith A, Vandenberghe R, et al. Automated quantification of 18F-flutemetamol PET activity for categorizing scans as negative or positive for brain amyloid: concordance with visual image reads. J Nucl Med. 2014; 55:1623–1628.
Article24. Rousset OG, Ma Y, Evans AC. Correction for partial volume effects in PET: principle and validation. J Nucl Med. 1998; 39:904–911.25. Rousset OG, Collins DL, Rahmim A, Wong DF. Design and implementation of an automated partial volume correction in PET: application to dopamine receptor quantification in the normal human striatum. J Nucl Med. 2008; 49:1097–1106.
Article26. Seelaar H, Kamphorst W, Rosso SM, Azmani A, Masdjedi R, de Koning I, et al. Distinct genetic forms of frontotemporal dementia. Neurology. 2008; 71:1220–1226.
Article27. Mesulam M, Wicklund A, Johnson N, Rogalski E, Léger GC, Rademaker A, et al. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann Neurol. 2008; 63:709–719.
Article28. Josephs KA, Petersen RC, Knopman DS, Boeve BF, Whitwell JL, Duffy JR, et al. Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology. 2006; 66:41–48.
Article29. Kikuchi A, Okamura N, Hasegawa T, Harada R, Watanuki S, Funaki Y, et al. In vivo visualization of tau deposits in corticobasal syndrome by 18F-THK5351 PET. Neurology. 2016; 87:2309–2316.
Article30. Vettermann F, Brendel M, Danek A, Levin J, Bartenstein P, Okamura N, et al. [18F] THK-5351 PET in patients with clinically diagnosed progressive supranuclear palsy. J Nucl Med. 2016; 57:457.31. Ishiki A, Harada R, Okamura N, Tomita N, Rowe CC, Villemagne VL, et al. Tau imaging with [18F]THK-5351 in progressive supranuclear palsy. Eur J Neurol. 2017; 24:130–136.
Article32. Gorno-Tempini ML, Ogar JM, Brambati SM, Wang P, Jeong JH, Rankin KP, et al. Anatomical correlates of early mutism in progressive nonfluent aphasia. Neurology. 2006; 67:1849–1851.
Article33. Kremen SA, Mendez MF, Tsai PH, Teng E. Extrapyramidal signs in the primary progressive aphasias. Am J Alzheimers Dis Other Demen. 2011; 26:72–77.
Article34. Ferrari J, Pontello N, Martinez-Cuitiño M, Borovinsky G, Gleichgerrcht E, Torralva T, et al. Extrapyramidal signs across variants of primary progressive aphasias. Mov Disord. 2014; 29 Suppl 1:598.35. Gulyás B, Pavlova E, Kása P, Gulya K, Bakota L, Várszegi S, et al. Activated MAO-B in the brain of Alzheimer patients, demonstrated by [11C]-L-deprenyl using whole hemisphere autoradiography. Neurochem Int. 2011; 58:60–68.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Relationships between [¹â¸F]-THK5351 Retention and Language Functions in Primary Progressive Aphasia
- Corticobasal Degeneration Presenting as Non-Fluent/Agrammatic Primary Progressive Aphasia: A Case Report
- Progressive Nonfluent Aphasia With Ideomotor Apraxia and Rigidity in the Right Upper Extremity
- Case Series of Right-Hemisphere Nonfluent Variant of Primary Progressive Aphasia
- Longitudinal Clinical Changes of Non-Fluent/Agrammatic Primary Progressive Aphasia as Tau Spectrum Disorder: A Case Report