Diabetes Metab J.
2019 Apr;43(2):222-235. 10.4093/dmj.2018.0020.
Increased Nociceptive Responses in Streptozotocin-Induced Diabetic Rats and the Related Expression of Spinal NR2B Subunit of N-Methyl-D-Aspartate Receptors
- Affiliations
-
- 1School of Health Sciences, Universiti Sains Malaysia Health Campus, Kota Bharu, Malaysia. idriskk@usm.my
- 2Physiology Department, School of Medical Sciences, Universiti Sains Malaysia Health Campus, Kota Bharu, Malaysia.
- KMID: 2442774
- DOI: http://doi.org/10.4093/dmj.2018.0020
Abstract
- BACKGROUND
This study investigated the role of NR2B in a modulated pain process in the painful diabetic neuropathy (PDN) rat using various pain stimuli.
METHODS
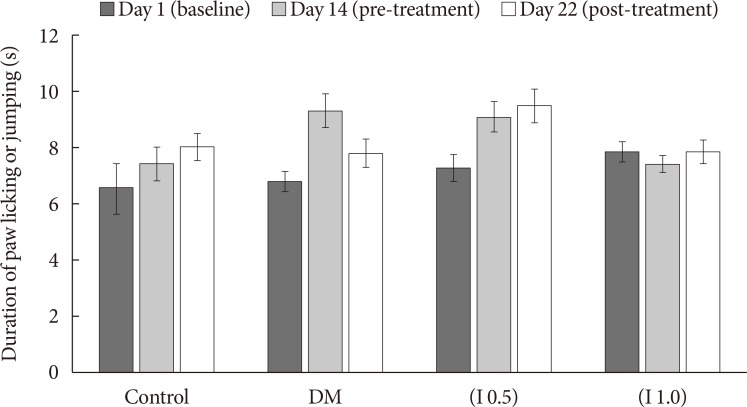
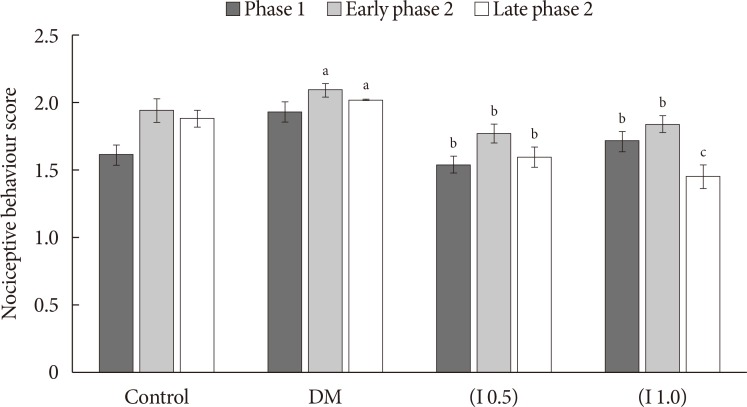
Thirty-two Sprague-Dawley male rats were randomly allocated into four groups (n=8): control, diabetes mellitus (DM) rats and diabetic rats treated with ifenprodil at a lower dose (0.5 µg/day) (I 0.5) or higher dose (1.0 µg/day) (I 1.0). DM was induced by a single injection of streptozotocin at 60 mg/kg on day 0 of experimentation. Diabetic status was assessed on day 3 of the experimentation. The responses on both tactile and thermal stimuli were assessed on day 0 (baseline), day 14 (pre-intervention), and day 22 (post-intervention). Ifenprodil was given intrathecally for 7 days from day 15 until day 21. On day 23, 5% formalin was injected into the rats' hind paw and the nociceptive responses were recorded for 1 hour. The rats were sacrificed 72 hours post-formalin injection and an analysis of the spinal NR2B expression was performed.
RESULTS
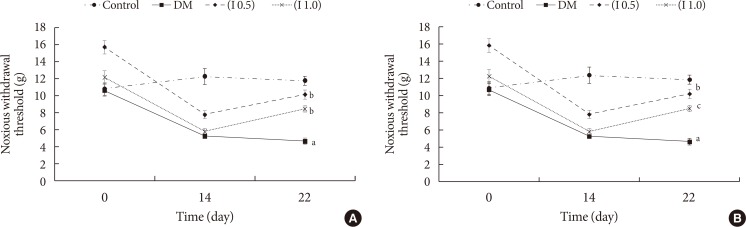
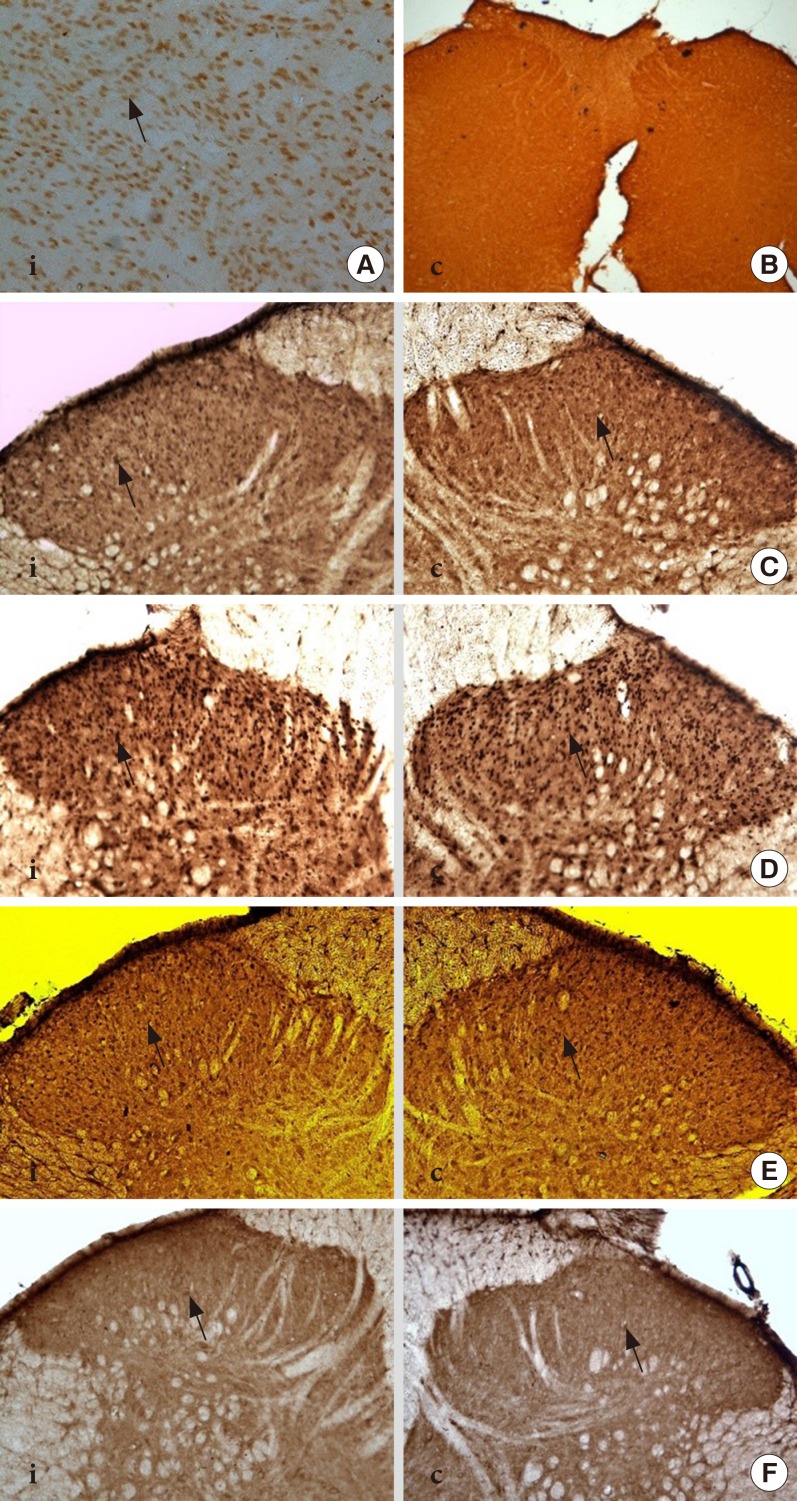
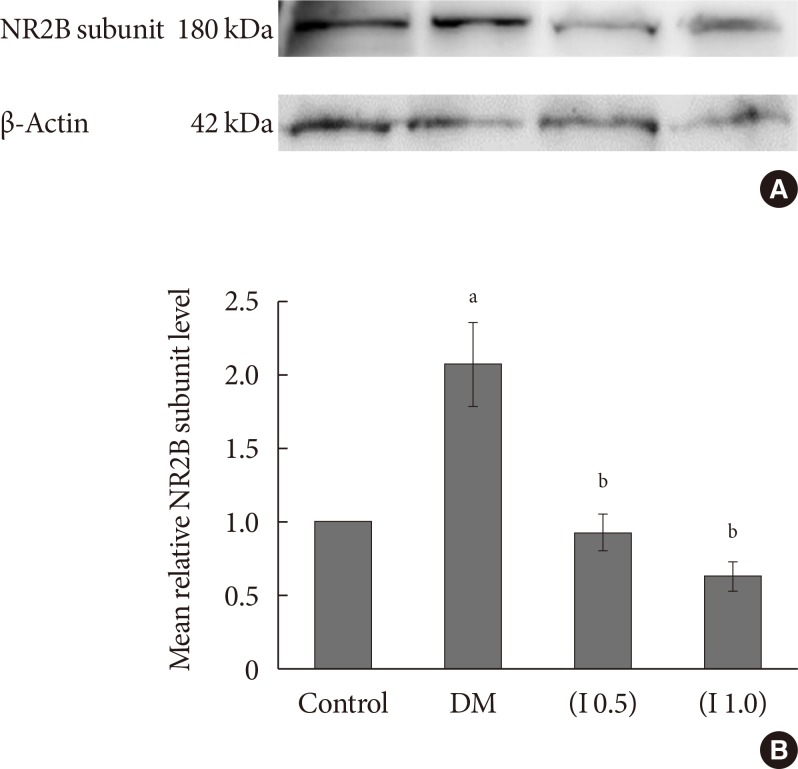
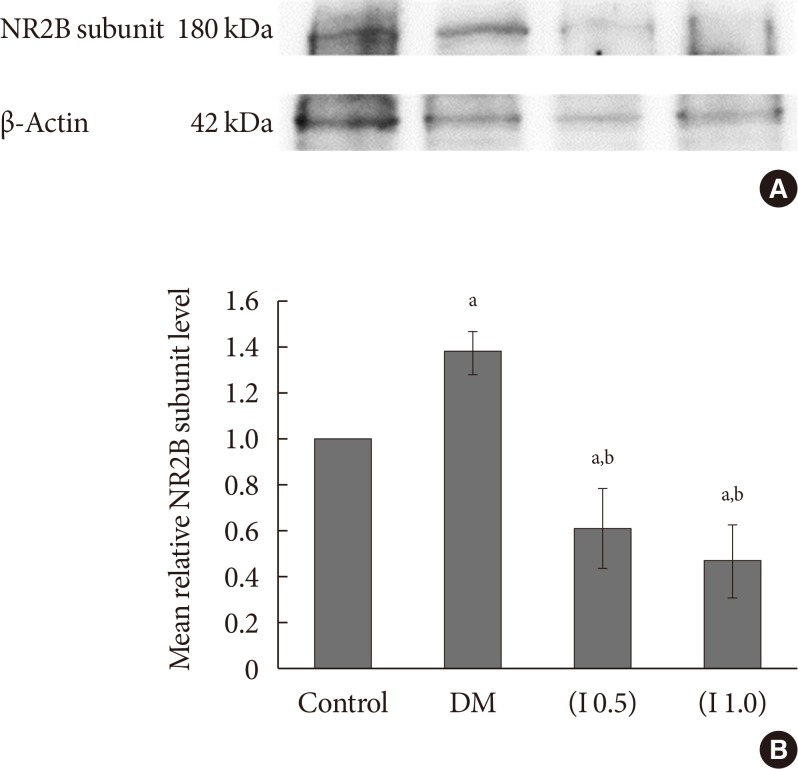
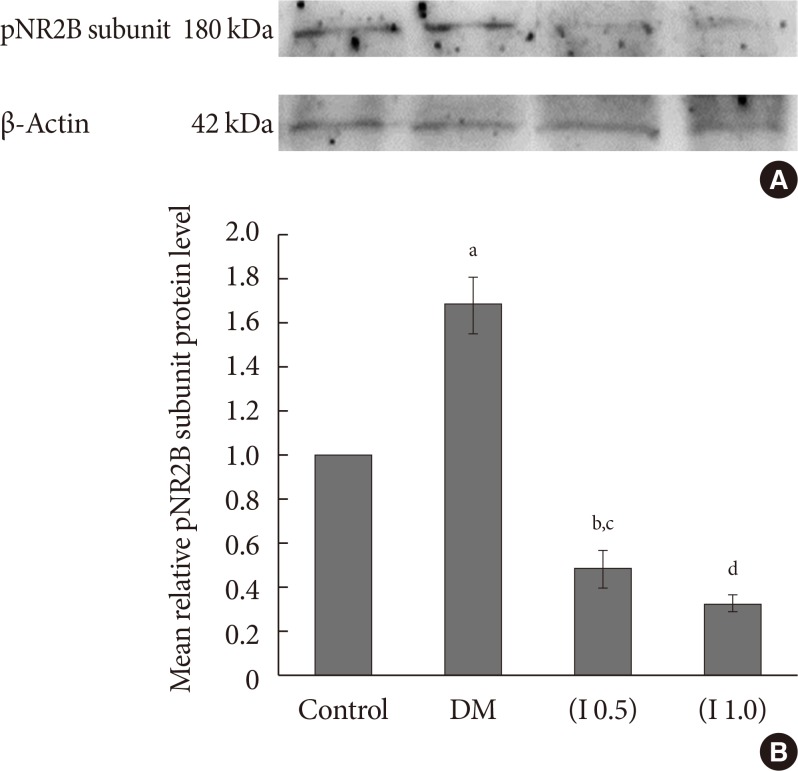
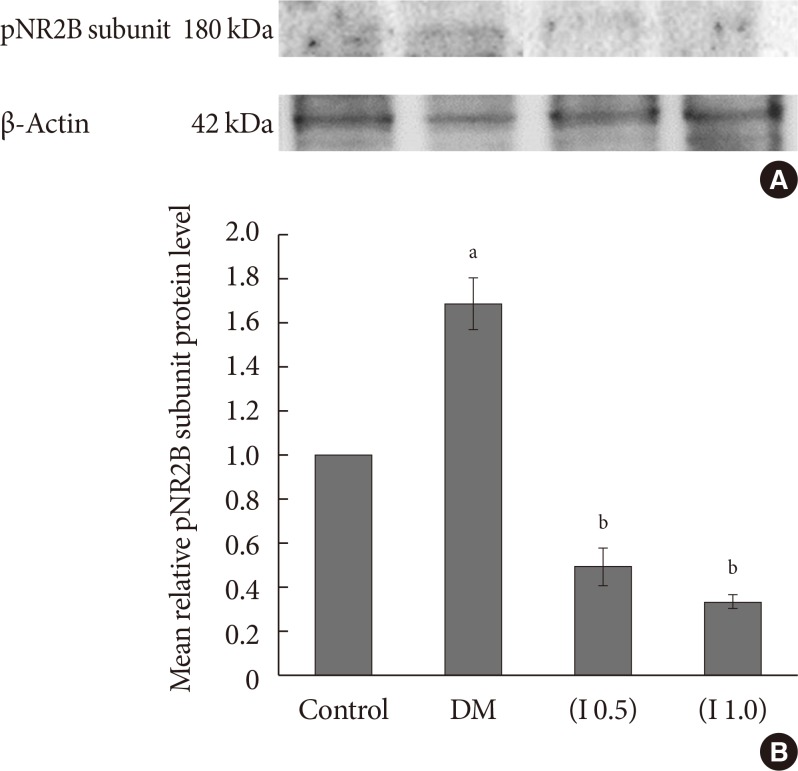
DM rats showed a significant reduction in pain threshold in response to the tactile and thermal stimuli and higher nociceptive response during the formalin test accompanied by the higher expression of phosphorylated spinal NR2B in both sides of the spinal cord. Ifenprodil treatment for both doses showed anti-allodynic and anti-nociceptive effects with lower expression of phosphorylated and total spinal NR2B.
CONCLUSION
We suggest that the pain process in the streptozotocin-induced diabetic rat that has been modulated is associated with the higher phosphorylation of the spinal NR2B expression in the development of PDN, which is similar to other models of neuropathic rats.
MeSH Terms
Figure
Reference
-
1. Stogdale L. Definition of diabetes mellitus. Cornell Vet. 1986; 76:156–174. PMID: 3516569.2. Zochodne DW, Malik RA. Chapter 5, Painful diabetic neuropathy: clinical aspects. Handbook of clinical neurology: diabetes and the nervous system. Amsterdam: Elsevier;2014. p. 53–61.3. Calcutt NA, Backonja MM. Pathogenesis of pain in peripheral diabetic neuropathy. Curr Diab Rep. 2007; 7:429–434. PMID: 18255005.
Article4. Wu HY, Hsu FC, Gleichman AJ, Baconguis I, Coulter DA, Lynch DR. Fyn-mediated phosphorylation of NR2B Tyr-1336 controls calpain-mediated NR2B cleavage in neurons and heterologous systems. J Biol Chem. 2007; 282:20075–20087. PMID: 17526495.
Article5. Loftis JM, Janowsky A. The N-methyl-D-aspartate receptor subunit NR2B: localization, functional properties, regulation, and clinical implications. Pharmacol Ther. 2003; 97:55–85. PMID: 12493535.
Article6. Qu XX, Cai J, Li MJ, Chi YN, Liao FF, Liu FY, Wan Y, Han JS, Xing GG. Role of the spinal cord NR2B-containing NMDA receptors in the development of neuropathic pain. Exp Neurol. 2009; 215:298–307. PMID: 19046970.
Article7. Nagy GG, Watanabe M, Fukaya M, Todd AJ. Synaptic distribution of the NR1, NR2A and NR2B subunits of the N-methyl-d-aspartate receptor in the rat lumbar spinal cord revealed with an antigen-unmasking technique. Eur J Neurosci. 2004; 20:3301–3312. PMID: 15610162.
Article8. Guo W, Zou S, Guan Y, Ikeda T, Tal M, Dubner R, Ren K. Tyrosine phosphorylation of the NR2B subunit of the NMDA receptor in the spinal cord during the development and maintenance of inflammatory hyperalgesia. J Neurosci. 2002; 22:6208–6217. PMID: 12122079.
Article9. Wei F, Wang GD, Kerchner GA, Kim SJ, Xu HM, Chen ZF, Zhuo M. Genetic enhancement of inflammatory pain by forebrain NR2B overexpression. Nat Neurosci. 2001; 4:164–169. PMID: 11175877.
Article10. Miki K, Zhou QQ, Guo W, Guan Y, Terayama R, Dubner R, Ren K. Changes in gene expression and neuronal phenotype in brain stem pain modulatory circuitry after inflammation. J Neurophysiol. 2002; 87:750–760. PMID: 11826044.
Article11. Williams K. Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol Pharmacol. 1993; 44:851–859. PMID: 7901753.12. Chizh BA, Reissmuller E, Schlutz H, Scheede M, Haase G, Englberger W. Supraspinal vs spinal sites of the antinociceptive action of the subtype-selective NMDA antagonist ifenprodil. Neuropharmacology. 2001; 40:212–220. PMID: 11114400.
Article13. Chizh BA, Headley PM, Tzschentke TM. NMDA receptor antagonists as analgesics: focus on the NR2B subtype. Trends Pharmacol Sci. 2001; 22:636–642. PMID: 11730974.
Article14. Boyce S, Wyatt A, Webb JK, O’Donnell R, Mason G, Rigby M, Sirinathsinghji D, Hill RG, Rupniak NM. Selective NMDA NR2B antagonists induce antinociception without motor dysfunction: correlation with restricted localisation of NR2B subunit in dorsal horn. Neuropharmacology. 1999; 38:611–623. PMID: 10340299.
Article15. Zulazmi NA, Gopalsamy B, Farouk AA, Sulaiman MR, Bharatham BH, Perimal EK. Antiallodynic and antihyperalgesic effects of zerumbone on a mouse model of chronic constriction injury-induced neuropathic pain. Fitoterapia. 2015; 105:215–221. PMID: 26205045.
Article16. Daulhac L, Maffre V, Mallet C, Etienne M, Privat AM, Kowalski-Chauvel A, Seva C, Fialip J, Eschalier A. Phosphorylation of spinal N-methyl-D-aspartate receptor NR1 subunits by extracellular signal-regulated kinase in dorsal horn neurons and microglia contributes to diabetes-induced painful neuropathy. Eur J Pain. 2011; 15:169. PMID: 20594879.17. Ma C. Animal models of pain. Int Anesthesiol Clin. 2007; 45:121–131.
Article18. Lu R, Schmidtko A. Direct intrathecal drug delivery in mice for detecting in vivo effects of cGMP on pain processing. Methods Mol Biol. 2013; 1020:215–221. PMID: 23709036.
Article19. Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977; 4:161–174. PMID: 564014.
Article20. Fu KY, Light AR, Matsushima GK, Maixner W. Microglial reactions after subcutaneous formalin injection into the rat hind paw. Brain Res. 1999; 825:59–67. PMID: 10216173.
Article21. Stanley BG, Urstadt KR, Charles JR, Kee T. Glutamate and GABA in lateral hypothalamic mechanisms controlling food intake. Physiol Behav. 2011; 104:40–46. PMID: 21550353.
Article22. Khan AM, Curras MC, Dao J, Jamal FA, Turkowski CA, Goel RK, Gillard ER, Wolfsohn SD, Stanley BG. Lateral hypothalamic NMDA receptor subunits NR2A and/or NR2B mediate eating: immunochemical/behavioral evidence. Am J Physiol. 1999; 276(3 Pt 2):R880–R891. PMID: 10070151.23. Anson JA. Feeding in response to site-selective antagonists of hindbrain NMDA receptors [dissertation]. Pullman: University Honors College, Washington State University;2004.24. Zhang W, Shi CX, Gu XP, Ma ZL, Zhu W. Ifenprodil induced antinociception and decreased the expression of NR2B subunits in the dorsal horn after chronic dorsal root ganglia compression in rats. Anesth Analg. 2009; 108:1015–1020. PMID: 19224818.
Article25. Bolay H, Moskowitz MA. Mechanisms of pain modulation in chronic syndromes. Neurology. 2002; 59(5 Suppl 2):S2–S7.
Article26. Sung B, Na HS, Kim YI, Yoon YW, Han HC, Nahm SH, Hong SK. Supraspinal involvement in the production of mechanical allodynia by spinal nerve injury in rats. Neurosci Lett. 1998; 246:117–119. PMID: 9627194.
Article27. Malcangio M, Tomlinson DR. A pharmacologic analysis of mechanical hyperalgesia in streptozotocin/diabetic rats. Pain. 1998; 76:151–157. PMID: 9696468.
Article28. Khan GM, Chen SR, Pan HL. Role of primary afferent nerves in allodynia caused by diabetic neuropathy in rats. Neuroscience. 2002; 114:291–299. PMID: 12204199.
Article29. Anjaneyulu M, Chopra K. Quercetin, a bioflavonoid, attenuates thermal hyperalgesia in a mouse model of diabetic neuropathic pain. Prog Neuropsychopharmacol Biol Psychiatry. 2003; 27:1001–1005. PMID: 14499317.
Article30. Sharma S, Kulkarni SK, Agrewala JN, Chopra K. Curcumin attenuates thermal hyperalgesia in a diabetic mouse model of neuropathic pain. Eur J Pharmacol. 2006; 536:256–261. PMID: 16584726.
Article31. DeLeo JA, Coombs DW, Willenbring S, Colburn RW, Fromm C, Wagner R, Twitchell BB. Characterization of a neuropathic pain model: sciatic cryoneurolysis in the rat. Pain. 1994; 56:9–16. PMID: 8159445.
Article32. Pabreja K, Dua K, Sharma S, Padi SS, Kulkarni SK. Minocycline attenuates the development of diabetic neuropathic pain: possible anti-inflammatory and anti-oxidant mechanisms. Eur J Pharmacol. 2011; 661:15–21. PMID: 21536024.
Article33. Besson JM, Chaouch A. Peripheral and spinal mechanisms of nociception. Physiol Rev. 1987; 67:67–186. PMID: 3543978.
Article34. Diemel LT, Stevens EJ, Willars GB, Tomlinson DR. Depletion of substance P and calcitonin gene-related peptide in sciatic nerve of rats with experimental diabetes; effects of insulin and aldose reductase inhibition. Neurosci Lett. 1992; 137:253–256. PMID: 1374869.
Article35. Hayati AA, Zalina I, Myo T, Badariah AA, Azhar A, Idris L. Modulation of formalin-induced fos-like immunoreactivity in the spinal cord by swim stress-induced analgesia, morphine and ketamine. Ger Med Sci. 2008; 6:Doc05. PMID: 19675733.36. Fu KY, Light AR, Maixner W. Long-lasting inflammation and long-term hyperalgesia after subcutaneous formalin injection into the rat hindpaw. J Pain. 2001; 2:2–11. PMID: 14622781.
Article37. Courteix C, Eschalier A, Lavarenne J. Streptozocin-induced diabetic rats: behavioural evidence for a model of chronic pain. Pain. 1993; 53:81–88. PMID: 8316394.
Article38. Ma QP, Hargreaves RJ. Localization of N-methyl-D-aspartate NR2B subunits on primary sensory neurons that give rise to small-caliber sciatic nerve fibers in rats. Neuroscience. 2000; 101:699–707. PMID: 11113318.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Changes of NR2B and c-fos mRNA Expressionin Rat Hippocampal CA1 Neuronal ChangeFollowing Hypoxia and Reoxygenation
- Effect of Developmental Lead Exposure on the Expression of Hippocampal NMDA Receptor Subunit mRNA
- Combined actions of Na+/K+-ATPase, NCX1 and glutamate dependent NMDA receptors in ischemic rat brain penumbra
- A Study for the Expression of the N-Methyl-D-Aspartate (NMDA) Subunit 2A(NR2A) and 2B(NR2B) of Rat Hippocampal Slices in the Hypoxic State
- The ontogeny of excitatory amino acid receptors in the rat brain quantitative autoradiographic study: I. N-methyl-D-aspartate receptors