J Periodontal Implant Sci.
2012 Dec;42(6):217-223.
Bacterial adhesion and colonization differences between zirconia and titanium implant abutments: an in vivo human study
- Affiliations
-
- 1Department of Oral and Maxillofacial Surgery, State University of West Parana, Cascavel, Brazil.
- 2Division of Oral and Maxillofacial Surgery, Department of Oral Diagnosis, State University of Campinas Pracicaba Dental School, Piracicaba, Brazil.
- 3Division of Oral and Maxillofacial Surgery, Department of Dentristry, University of La Frontera School of Medicine, Temuco, Chile. solate@ufro.cl
Abstract
- PURPOSE
Several parameters have been described for determining the success or failure of dental implants. The surface properties of transgingival implant components have had a great impact on the long-term success of dental implants. The purpose of this study was to compare the tendency of two periodontal pathogens to adhere to and colonize zirconia abutments and titanium alloys both in hard surfaces and soft tissues.
METHODS
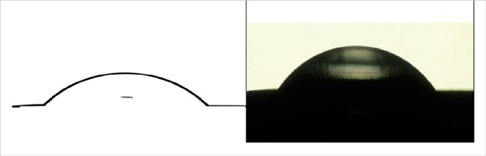
Twelve patients participated in this study. Three months after implant placement, the abutments were connected. Five weeks following the abutment connections, the abutments were removed, probing depth measurements were recorded, and gingival biopsies were performed. The abutments and gingival biopsies taken from the buccal gingiva were analyzed using real-time polymerase chain reaction to compare the DNA copy numbers of Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, and total bacteria. The surface free energy of the abutments was calculated using the sessile water drop method before replacement. Data analyses used the Mann Whitney U-test, and P-values below 0.05 find statistical significance.
RESULTS
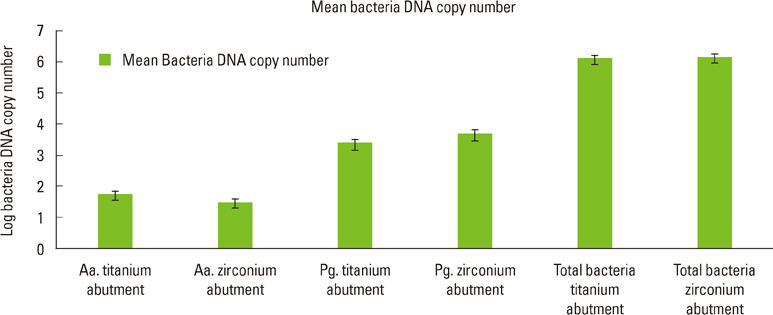
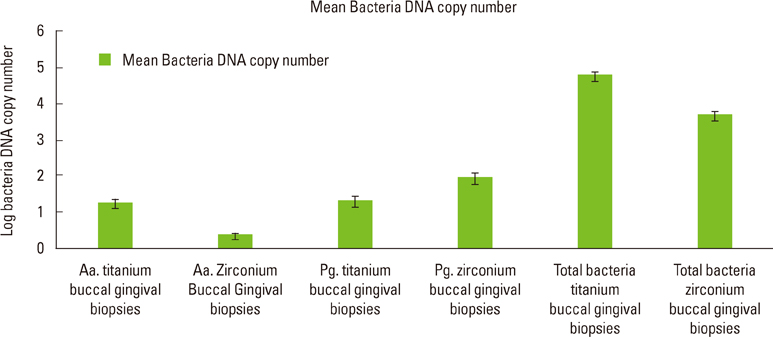
The present study showed no statistically significant differences between the DNA copy numbers of A. actinomycetemcomitans, P. gingivalis, and total bacteria for both the titanium and zirconia abutments and the biopsies taken from their buccal gingiva. The differences between the free surface energy of the abutments had no influence on the microbiological findings.
CONCLUSIONS
Zirconia surfaces have comparable properties to titanium alloy surfaces and may be suitable and safe materials for the long-term success of dental implants.
Keyword
MeSH Terms
Figure
Reference
-
1. Branemark PI, Hansson BO, Adell R, Breine U, Lindstrom J, Hallen O, et al. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand J Plast Reconstr Surg Suppl. 1977. 16:1–132.2. Buser D, Mericske-Stern R, Bernard JP, Behneke A, Behneke N, Hirt HP, et al. Long-term evaluation of non-submerged ITI implants. Part 1: 8-year life table analysis of a prospective multi-center study with 2359 implants. Clin Oral Implants Res. 1997. 8:161–172.
Article3. Buser D, Weber HP, Lang NP. Tissue integration of non-submerged implants. 1-year results of a prospective study with 100 ITI hollow-cylinder and hollow-screw implants. Clin Oral Implants Res. 1990. 1:33–40.
Article4. Heijdenrijk K, Raghoebar GM, Meijer HJ, Stegenga B, van der Reijden WA. Feasibility and influence of the microgap of two implants placed in a non-submerged procedure: a five-year follow-up clinical trial. J Periodontol. 2006. 77:1051–1060.
Article5. Oh TJ, Yoon J, Misch CE, Wang HL. The causes of early implant bone loss: myth or science? J Periodontol. 2002. 73:322–333.
Article6. Lindhe J, Berglundh T, Ericsson I, Liljenberg B, Marinello C. Experimental breakdown of peri-implant and periodontal tissues. A study in the beagle dog. Clin Oral Implants Res. 1992. 3:9–16.
Article7. Lang NP, Bragger U, Walther D, Beamer B, Kornman KS. Ligature-induced peri-implant infection in cynomolgus monkeys. I. Clinical and radiographic findings. Clin Oral Implants Res. 1993. 4:2–11.
Article8. Becker W, Becker BE, Newman MG, Nyman S. Clinical and microbiologic findings that may contribute to dental implant failure. Int J Oral Maxillofac Implants. 1990. 5:31–38.9. Sumida S, Ishihara K, Kishi M, Okuda K. Transmission of periodontal disease-associated bacteria from teeth to osseointegrated implant regions. Int J Oral Maxillofac Implants. 2002. 17:696–702.10. Grossner-Schreiber B, Griepentrog M, Haustein I, Muller WD, Lange KP, Briedigkeit H, et al. Plaque formation on surface modified dental implants. An in vitro study. Clin Oral Implants Res. 2001. 12:543–551.11. Bollen CM, Papaioanno W, Van Eldere J, Schepers E, Quirynen M, van Steenberghe D. The influence of abutment surface roughness on plaque accumulation and peri-implant mucositis. Clin Oral Implants Res. 1996. 7:201–211.
Article12. Binon PP. Implants and components: entering the new millennium. Int J Oral Maxillofac Implants. 2000. 15:76–94.13. Rimondini L, Cerroni L, Carrassi A, Torricelli P. Bacterial colonization of zirconia ceramic surfaces: an in vitro and in vivo study. Int J Oral Maxillofac Implants. 2002. 17:793–798.14. Lautenschlager EP, Monaghan P. Titanium and titanium alloys as dental materials. Int Dent J. 1993. 43:245–253.15. Steinberg D, Sela MN, Klinger A, Kohavi D. Adhesion of periodontal bacteria to titanium, and titanium alloy powders. Clin Oral Implants Res. 1998. 9:67–72.
Article16. Piconi C, Maccauro G. Zirconia as a ceramic biomaterial. Biomaterials. 1999. 20:1–25.
Article17. Scarano A, Piattelli M, Caputi S, Favero GA, Piattelli A. Bacterial adhesion on commercially pure titanium and zirconium oxide disks: an in vivo human study. J Periodontol. 2004. 75:292–296.
Article18. Heuer W, Elter C, Demling A, Neumann A, Suerbaum S, Hannig M, et al. Analysis of early biofilm formation on oral implants in man. J Oral Rehabil. 2007. 34:377–382.
Article19. Consensus report. Periodontal diseases: pathogenesis and microbial factors. Ann Periodontol. 1996. 1:926–932.20. van Winkelhoff AJ, Goene RJ, Benschop C, Folmer T. Early colonization of dental implants by putative periodontal pathogens in partially edentulous patients. Clin Oral Implants Res. 2000. 11:511–520.
Article21. Rudney JD, Chen R, Sedgewick GJ. Intracellular Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in buccal epithelial cells collected from human subjects. Infect Immun. 2001. 69:2700–2707.
Article22. Image J [Internet]. c2012. 2012 Apr 4. Bethesda: National Institutes of Health;Available from: http://rsbweb.nih.gov/ij/.23. van Oss CJ. Interfacial forces in aqueous media. 2006. 2nd ed. Boca Raton: Talyor & Francis.24. Wei YH, Lai HC, Chen SY, Yeh MS, Chang JS. Biosurfactant production by Serratia marcescens SS-1 and its isogenic strain SMdeltaR defective in SpnR, a quorum-sensing LuxR family protein. Biotechnol Lett. 2004. 26:799–802.
Article25. Lau L, Sanz M, Herrera D, Morillo JM, Martin C, Silva A. Quantitative real-time polymerase chain reaction versus culture: a comparison between two methods for the detection and quantification of Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis and Tannerella forsythensis in subgingival plaque samples. J Clin Periodontol. 2004. 31:1061–1069.
Article26. Loe H, Theilade E, Jensen SB. Experimental gingivitis in man. J Periodontol. 1965. 36:177–187.
Article27. Hickey JS, O'Neal RB, Scheidt MJ, Strong SL, Turgeon D, Van Dyke TE. Microbiologic characterization of ligature-induced peri-implantitis in the microswine model. J Periodontol. 1991. 62:548–553.
Article28. Berglundh T, Lindhe J, Marinello C, Ericsson I, Liljenberg B. Soft tissue reaction to de novo plaque formation on implants and teeth. An experimental study in the dog. Clin Oral Implants Res. 1992. 3:1–8.
Article29. Quirynen M, Marechal M, Busscher HJ, Weerkamp AH, Darius PL, van Steenberghe D. The influence of surface free energy and surface roughness on early plaque formation. An in vivo study in man. J Clin Periodontol. 1990. 17:138–144.
Article30. Quirynen M, van der Mei HC, Bollen CM, Schotte A, Marechal M, Doornbusch GI, et al. An in vivo study of the influence of the surface roughness of implants on the microbiology of supra- and subgingival plaque. J Dent Res. 1993. 72:1304–1309.
Article31. Guilherme AS, Henriques GE, Zavanelli RA, Mesquita MF. Surface roughness and fatigue performance of commercially pure titanium and Ti-6Al-4V alloy after different polishing protocols. J Prosthet Dent. 2005. 93:378–385.
Article32. Quirynen M, Van der Mei HC, Bollen CM, Van den Bossche LH, Doornbusch GI, van Steenberghe D, et al. The influence of surface-free energy on supra- and subgingival plaque microbiology. An in vivo study on implants. J Periodontol. 1994. 65:162–167.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of marginal and internal fit of zirconia abutments with titanium abutments in internal hexagonal implants
- Retraction: Bacterial adhesion and colonization differences between zirconia and titanium implant abutments: an in vivo human study
- Zirconia Abutment Fracture in the Anterior Region: Case Series
- Effect of coloring treatment of translucent zirconia on the masking ability of metal abutment
- The effect of a titanium socket with a zirconia abutment on screw loosening after thermocycling in an internally connected implant: a preliminary study