J Periodontal Implant Sci.
2012 Dec;42(6):185-195.
Anti-inflammatory effect of (-)-epigallocatechin-3-gallate on Porphyromonas gingivalis lipopolysaccharide-stimulated fibroblasts and stem cells derived from human periodontal ligament
- Affiliations
-
- 1Department of Periodontology, Research Institute for Periodontal Regeneration, Yonsei University College of Dentistry, Seoul, Korea. SHCHOI726@yuhs.ac
- 2Division of Periodontology, Department of Dentistry, Inha University School of Medicine, Incheon, Korea.
- 3Department of Oral Biology, Research Center for Orofacial Hard Tissue Regeneration, Yonsei University College of Dentistry, Seoul, Korea.
- 4Department of Periodontology, Pusan National University School of Dentistry, Yangsan, Korea.
Abstract
- PURPOSE
(-)-epigallocatechin-3-gallate (EGCG) has been reported to exert anti-inflammatory and antibacterial effects in periodontitis. However, its exact mechanism of action has yet to be determined. The present in vitro study evaluated the anti-inflammatory effects of EGCG on human periodontal ligament fibroblasts (hPDLFs) and human periodontal ligament stem cells (hPDLSCs) affected by bacterial lipopolysaccharide (LPS) extracted from Porphyromonas gingivalis.
METHODS
hPDLFs and hPDLSCs were extracted from healthy young adults and were treated with EGCG and/or P. gingivalis LPS. After 1, 3, 5, and 7 days from treatment, cytotoxic and proliferative effects were evaluated using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay and bromodeoxyuridine assay, respectively. And then, the gene expressions of hPDLFs and hPDLSCs were observed for interleukin (IL)-1beta, IL-6, tumor necrosis factor (TNF)-alpha, osteoprotegerin (OPG), receptor activator of nuclear factor kappa-B ligand (RANKL), and RANKL/OPG using real-time polymerase chain reaction (PCR) at 0, 6, 24, and 48 hours after treatment. The experiments were performed with the following groups for hPDLFs and hPDLSCs; 1) No treat, 2) EGCG alone, 3) P. gingivalis LPS alone, 4) EGCG+P. gingivalis LPS.
RESULTS
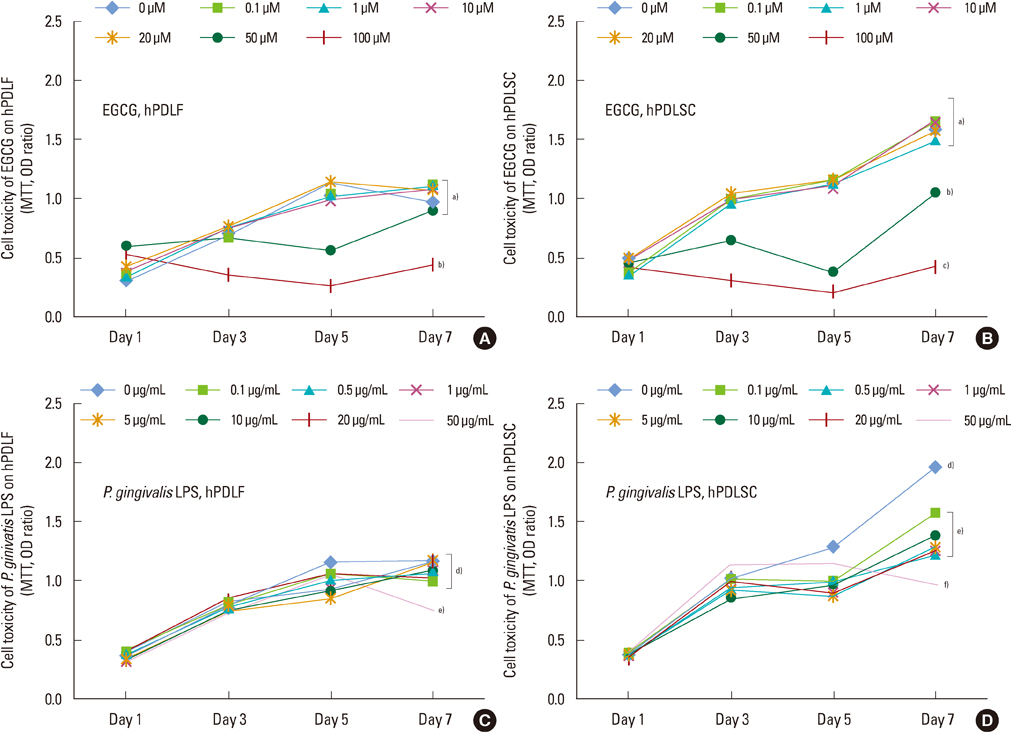
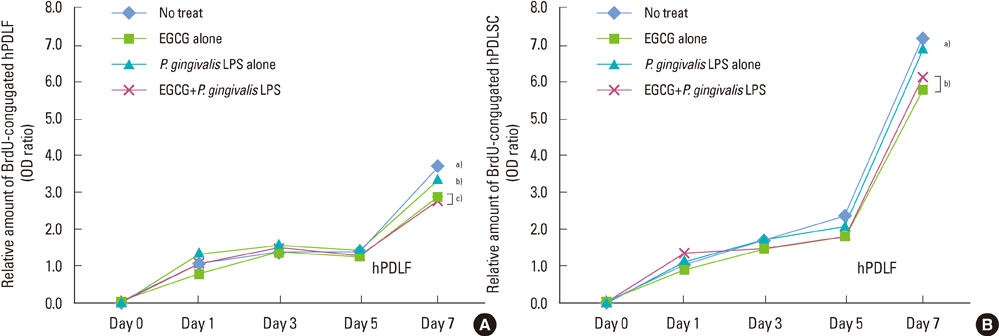
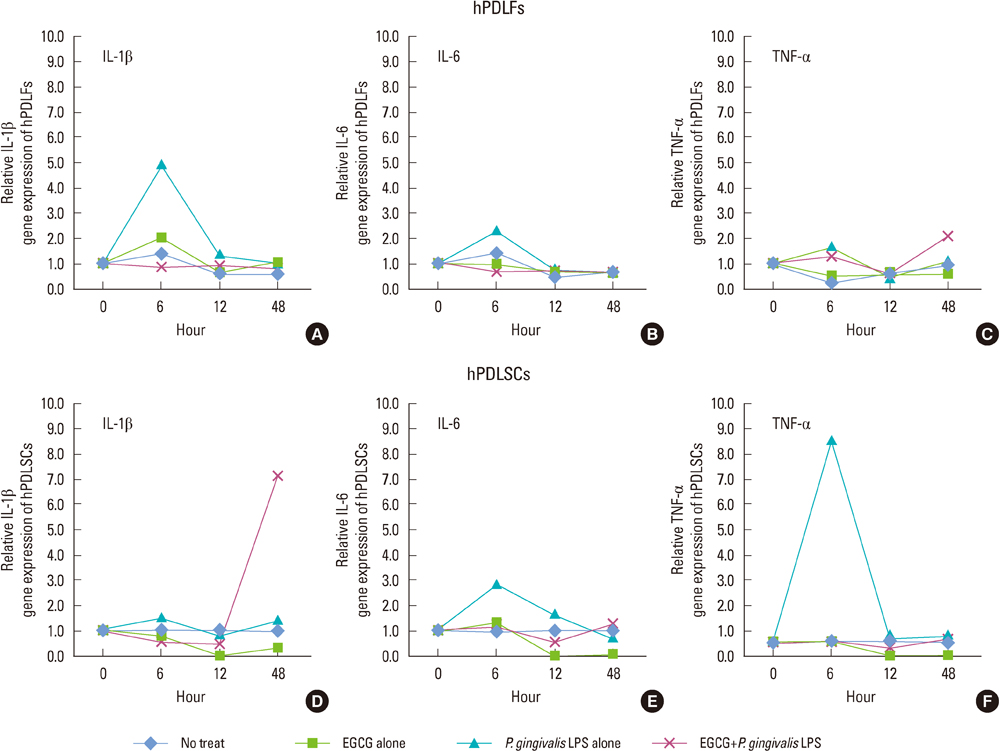
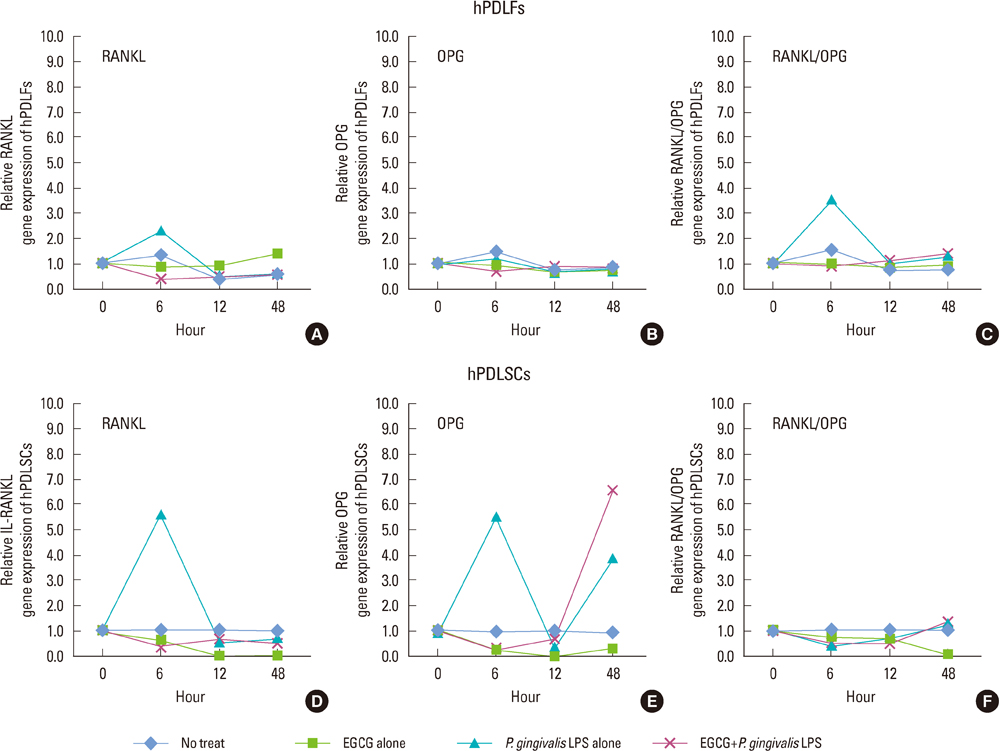
The 20 microM of EGCG and 20 microg/mL of P. gingivalis LPS had the lowest cytotoxic effects, so those concentrations were used for further experiments. The proliferations of hPDLFs and hPDLSCs increased in all groups, though the 'EGCG alone' showed less increase. In real-time PCR, the hPDLFs and hPDLSCs of 'EGCG alone' showed similar gene expressions to those cells of 'no treat'. The gene expressions of 'P. gingivalis LPS alone' in both hPDLFs and hPDLSCs were highly increased at 6 hours for IL-1beta, IL-6, TNF-alpha, RANKL, and RANKL/OPG, except the RANKL/OPG in hPDLSCs. However, those increased gene expressions were down-regulated in 'EGCG+P. gingivalis LPS' by the additional treatment of EGCG.
CONCLUSIONS
Our results demonstrate that EGCG could exert an anti-inflammatory effect in hPDLFs and hPDLSCs against a major pathogen of periodontitis, P. gingivalis LPS.
Keyword
MeSH Terms
-
Anti-Inflammatory Agents
Bromodeoxyuridine
Fibroblasts
Gene Expression
Humans
Interleukin-6
Interleukins
Osteoprotegerin
Periodontal Ligament
Periodontitis
Porphyromonas
Porphyromonas gingivalis
Real-Time Polymerase Chain Reaction
Stem Cells
Tetrazolium Salts
Thiazoles
Tumor Necrosis Factor-alpha
Young Adult
Anti-Inflammatory Agents
Bromodeoxyuridine
Interleukin-6
Interleukins
Osteoprotegerin
Tetrazolium Salts
Thiazoles
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Yamamoto T, Kita M, Oseko F, Nakamura T, Imanishi J, Kanamura N. Cytokine production in human periodontal ligament cells stimulated with Porphyromonas gingivalis. J Periodontal Res. 2006. 41:554–559.
Article2. Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004. 364:149–155.
Article3. Slots J, Bragd L, Wikstrom M, Dahlen G. The occurrence of Actinobacillus actinomycetemcomitans, Bacteroides gingivalis and Bacteroides intermedius in destructive periodontal disease in adults. J Clin Periodontol. 1986. 13:570–577.
Article4. Birkedal-Hansen H. Role of cytokines and inflammatory mediators in tissue destruction. J Periodontal Res. 1993. 28:500–510.
Article5. Nair SP, Meghji S, Wilson M, Reddi K, White P, Henderson B. Bacterially induced bone destruction: mechanisms and misconceptions. Infect Immun. 1996. 64:2371–2380.
Article6. Aznar C, Fitting C, Cavaillon JM. Lipopolysaccharide-induced production of cytokines by bone marrow-derived macrophages: dissociation between intracellular interleukin 1 production and interleukin 1 release. Cytokine. 1990. 2:259–265.
Article7. Boyce BF, Aufdemorte TB, Garrett IR, Yates AJ, Mundy GR. Effects of interleukin-1 on bone turnover in normal mice. Endocrinology. 1989. 125:1142–1150.
Article8. Gowen M, Wood DD, Ihrie EJ, McGuire MK, Russell RG. An interleukin 1 like factor stimulates bone resorption in vitro. Nature. 1983. 306:378–380.
Article9. Belibasakis GN, Bostanci N, Hashim A, Johansson A, Aduse-Opoku J, Curtis MA, et al. Regulation of RANKL and OPG gene expression in human gingival fibroblasts and periodontal ligament cells by Porphyromonas gingivalis: a putative role of the Arg-gingipains. Microb Pathog. 2007. 43:46–53.
Article10. Yun JH, Pang EK, Kim CS, Yoo YJ, Cho KS, Chai JK, et al. Inhibitory effects of green tea polyphenol (-)-epigallocatechin gallate on the expression of matrix metalloproteinase-9 and on the formation of osteoclasts. J Periodontal Res. 2004. 39:300–307.
Article11. Rogers J, Perkins I, van Olphen A, Burdash N, Klein TW, Friedman H. Epigallocatechin gallate modulates cytokine production by bone marrow-derived dendritic cells stimulated with lipopolysaccharide or muramyldipeptide, or infected with Legionella pneumophila. Exp Biol Med (Maywood). 2005. 230:645–651.
Article12. Yun JH, Kim CS, Cho KS, Chai JK, Kim CK, Choi SH. (-)-Epigallocatechin gallate induces apoptosis, via caspase activation, in osteoclasts differentiated from RAW 264.7 cells. J Periodontal Res. 2007. 42:212–218.
Article13. Lee YL, Hong CY, Kok SH, Hou KL, Lin YT, Chen MH, et al. An extract of green tea, epigallocatechin-3-gallate, reduces periapical lesions by inhibiting cysteine-rich 61 expression in osteoblasts. J Endod. 2009. 35:206–211.
Article14. Cabrera C, Artacho R, Gimenez R. Beneficial effects of green tea: a review. J Am Coll Nutr. 2006. 25:79–99.15. Crespy V, Williamson G. A review of the health effects of green tea catechins in in vivo animal models. J Nutr. 2004. 134:3431S–3440S.
Article16. Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003. 43:89–143.
Article17. Jung IH, Yun JH, Cho AR, Kim CS, Chung WG, Choi SH. Effect of (-)-epigallocatechin-3-gallate on maintaining the periodontal ligament cell viability of avulsed teeth: a preliminary study. J Periodontal Implant Sci. 2011. 41:10–16.
Article18. Sakanaka S, Aizawa M, Kim M, Yamamoto T. Inhibitory effects of green tea polyphenols on growth and cellular adherence of an oral bacterium, Porphyromonas gingivalis. Biosci Biotechnol Biochem. 1996. 60:745–749.
Article19. Hosokawa Y, Hosokawa I, Ozaki K, Nakanishi T, Nakae H, Matsuo T. Catechins inhibit CCL20 production in IL-17A-stimulated human gingival fibroblasts. Cell Physiol Biochem. 2009. 24:391–396.
Article20. Morrison DC, Leive L. Fractions of lipopolysaccharide from Escherichia coli O111:B4 prepared by two extraction procedures. J Biol Chem. 1975. 250:2911–2919.
Article21. Apicella MA. Isolation and characterization of lipopolysaccharides. Methods Mol Biol. 2008. 431:3–13.
Article22. Okada H, Murakami S. Cytokine expression in periodontal health and disease. Crit Rev Oral Biol Med. 1998. 9:248–266.
Article23. Wada N, Maeda H, Yoshimine Y, Akamine A. Lipopolysaccharide stimulates expression of osteoprotegerin and receptor activator of NF-kappa B ligand in periodontal ligament fibroblasts through the induction of interleukin-1 beta and tumor necrosis factor-alpha. Bone. 2004. 35:629–635.
Article24. Hirasawa M, Takada K, Makimura M, Otake S. Improvement of periodontal status by green tea catechin using a local delivery system: a clinical pilot study. J Periodontal Res. 2002. 37:433–438.
Article25. Ahmed S, Wang N, Lalonde M, Goldberg VM, Haqqi TM. Green tea polyphenol epigallocatechin-3-gallate (EGCG) differentially inhibits interleukin-1 beta-induced expression of matrix metalloproteinase-1 and -13 in human chondrocytes. J Pharmacol Exp Ther. 2004. 308:767–773.
Article26. Yang F, Oz HS, Barve S, de Villiers WJ, McClain CJ, Varilek GW. The green tea polyphenol (-)-epigallocatechin-3-gallate blocks nuclear factor-kappa B activation by inhibiting I kappa B kinase activity in the intestinal epithelial cell line IEC-6. Mol Pharmacol. 2001. 60:528–533.27. Liao S, Kao YH, Hiipakka RA. Green tea: biochemical and biological basis for health benefits. Vitam Horm. 2001. 62:1–94.
Article28. Bae JY, Kanamune J, Han DW, Matsumura K, Hyon SH. Reversible regulation of cell cycle-related genes by epigallocatechin gallate for hibernation of neonatal human tarsal fibroblasts. Cell Transplant. 2009. 18:459–469.
Article29. Han DW, Matsumura K, Kim B, Hyon SH. Time-dependent intracellular trafficking of FITC-conjugated epigallocatechin-3-O-gallate in L-929 cells. Bioorg Med Chem. 2008. 16:9652–9659.
Article30. Tang SN, Fu J, Shankar S, Srivastava RK. EGCG enhances the therapeutic potential of gemcitabine and CP690550 by inhibiting STAT3 signaling pathway in human pancreatic cancer. PLoS One. 2012. 7:e31067.
Article31. Pathirana RD, O'Brien-Simpson NM, Reynolds EC. Host immune responses to Porphyromonas gingivalis antigens. Periodontol 2000. 2010. 52:218–237.
Article32. Graves DT, Cochran D. The contribution of interleukin-1 and tumor necrosis factor to periodontal tissue destruction. J Periodontol. 2003. 74:391–401.
Article33. Ishimi Y, Miyaura C, Jin CH, Akatsu T, Abe E, Nakamura Y, et al. IL-6 is produced by osteoblasts and induces bone resorption. J Immunol. 1990. 145:3297–3303.34. Hasegawa T, Yoshimura Y, Kikuiri T, Yawaka Y, Takeyama S, Matsumoto A, et al. Expression of receptor activator of NF-kappa B ligand and osteoprotegerin in culture of human periodontal ligament cells. J Periodontal Res. 2002. 37:405–411.
Article35. Pfeilschifter J, Chenu C, Bird A, Mundy GR, Roodman GD. Interleukin-1 and tumor necrosis factor stimulate the formation of human osteoclastlike cells in vitro. J Bone Miner Res. 1989. 4:113–118.
Article36. Perez-Sayans M, Somoza-Martin JM, Barros-Angueira F, Rey JM, Garcia-Garcia A. RANK/RANKL/OPG role in distraction osteogenesis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010. 109:679–686.
Article37. Imatani T, Kato T, Okuda K. Production of inflammatory cytokines by human gingival fibroblasts stimulated by cell-surface preparations of Porphyromonas gingivalis. Oral Microbiol Immunol. 2001. 16:65–72.
Article38. Kim HS, Kim KH, Kim SH, Kim YS, Koo KT, Kim TI, et al. Immunomodulatory effect of canine periodontal ligament stem cells on allogenic and xenogenic peripheral blood mononuclear cells. J Periodontal Implant Sci. 2010. 40:265–270.
Article39. Nakanishi T, Mukai K, Yumoto H, Hirao K, Hosokawa Y, Matsuo T. Anti-inflammatory effect of catechin on cultured human dental pulp cells affected by bacteria-derived factors. Eur J Oral Sci. 2010. 118:145–150.
Article40. Hosokawa Y, Hosokawa I, Ozaki K, Nakanishi T, Nakae H, Matsuo T. Tea polyphenols inhibit IL-6 production in tumor necrosis factor superfamily 14-stimulated human gingival fibroblasts. Mol Nutr Food Res. 2010. 54:Suppl 2. S151–S158.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Gossypetin from Hibiscus sabdariffa on Interleukin-6 Production in Porphyromonas gingivalis Lipopolysaccharide-Stimulated Human Gingival Fibroblasts
- The Lipopolysaccharide from Porphyromonas gingivalis Induces Vascular Permeability
- The role of lysophosphatidic acid receptor 1 in inflammatory response induced by lipopolysaccharide from Porphyromonas gingivalis in human periodontal ligament stem cells
- Perturbation of host responses by Porphyromonas gingivalis biofilm
- Magnoliae Cortex and maize modulate Porphyromonas gingivalis-induced inflammatory reactions