Yonsei Med J.
2017 Mar;58(2):423-431. 10.3349/ymj.2017.58.2.423.
Role of TGFBIp in Wound Healing and Mucin Expression in Corneal Epithelial Cells
- Affiliations
-
- 1Department of Ophthalmology, Corneal Dystrophy Research Institute, Yonsei University College of Medicine, Seoul, Korea. eungkkim@yuhs.ac
- 2Institute of Vision Research, Severance Biomedical Science Institute, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2427133
- DOI: http://doi.org/10.3349/ymj.2017.58.2.423
Abstract
- PURPOSE
Transforming growth factor-β-induced protein (TGFBIp) is highly expressed in the cornea, and mutant TGFBIp induces corneal diseases. However, the function of TGFBIp in cornea epithelium is not fully investigated. Here, we tested the importance of TGFBIp in regulation of gene expression and corneal epithelial cell (CEC) activity.
MATERIALS AND METHODS
The effect of TGFBIp on CEC activity was analyzed by cell migration, adhesion, proliferation and wound healing assay. Analysis of gene expression was examined by western blot and quantitative reverse transcription PCR.
RESULTS
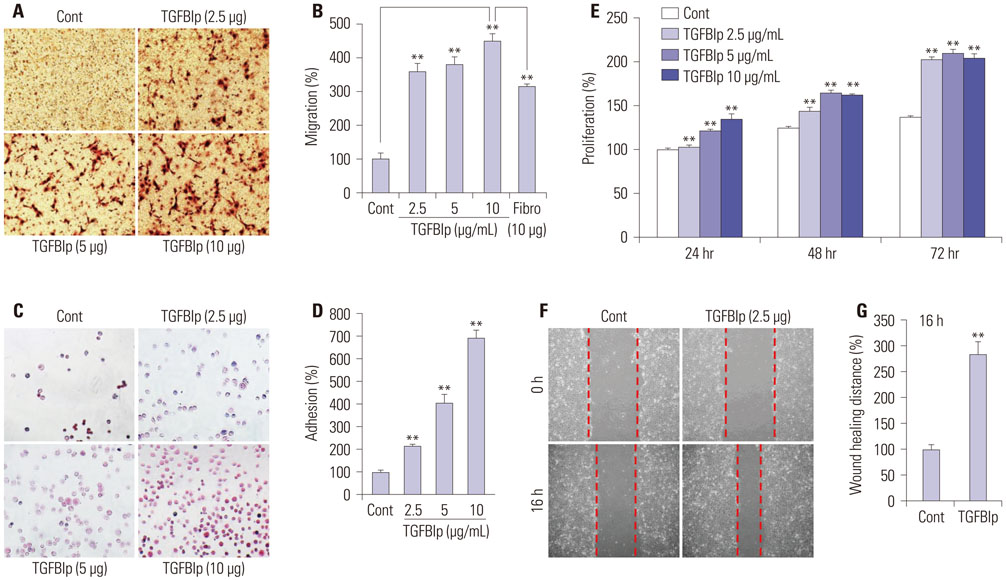
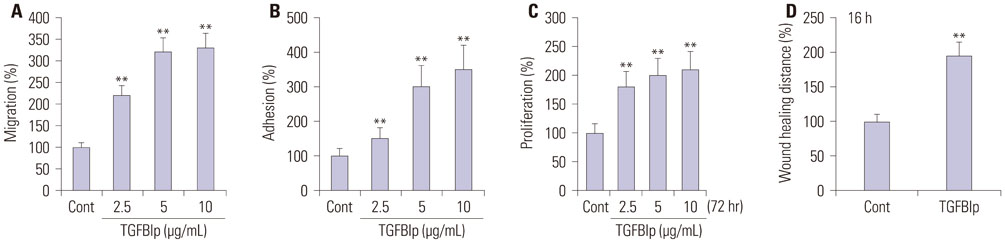
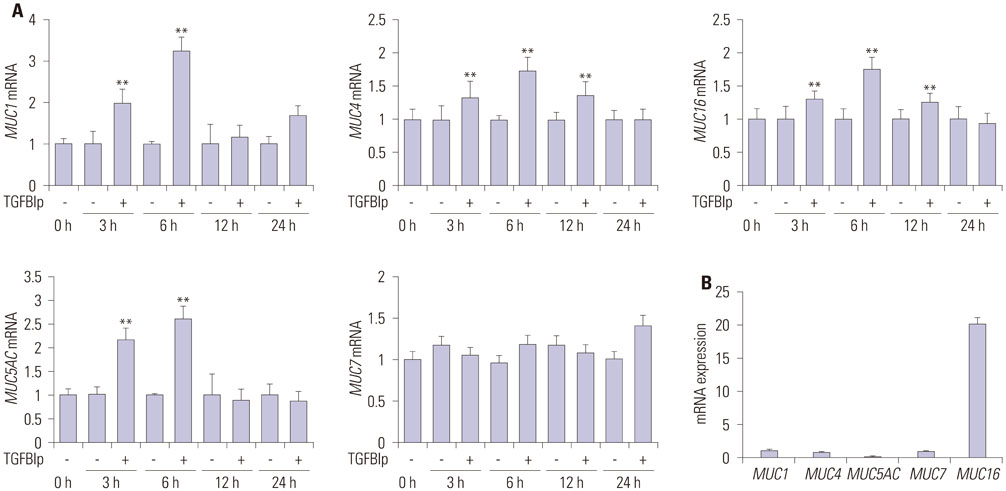
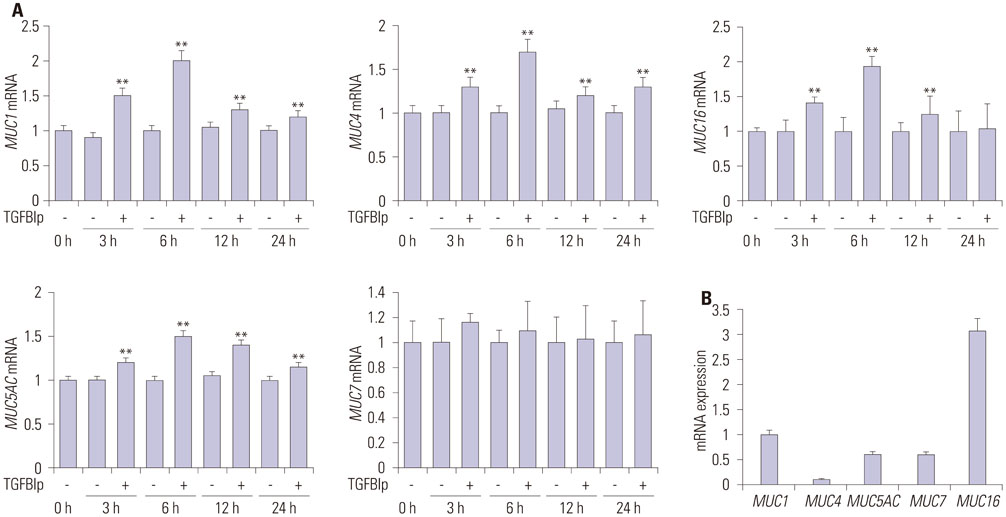
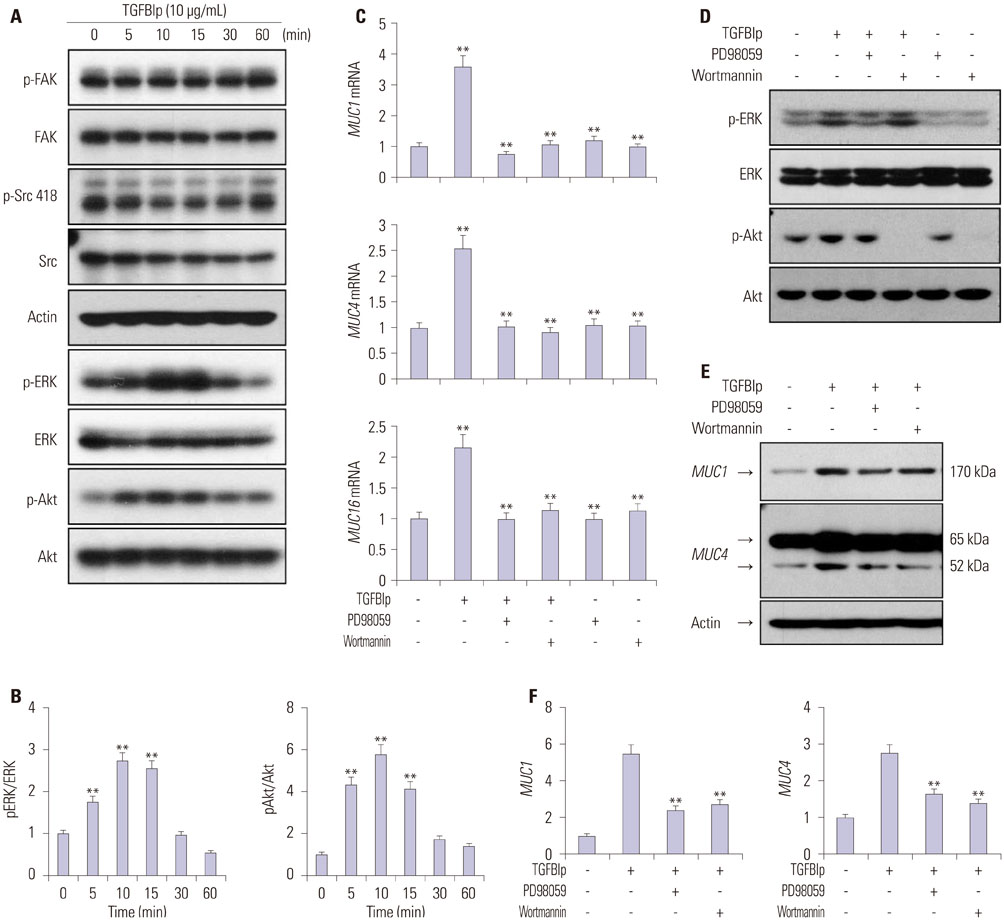
The results demonstrated that TGFBIp increased adhesion, migration, proliferation, and wound healing of CECs. Analysis of gene expression presented that TGFBIp-stimulated CECs exhibited increased expression of mucin family genes, such as MUC1, -4, -5AC, and -16. Furthermore, TGFBIp treatment increased the expression of MUC1, -4, -5AC, -7, and -16 in conjunctival epithelial cells. TGFBIp also increased the activity of intracellular signaling molecules ERK and AKT in CECs. Using pharmacologic inhibitors of ERK and AKT, we showed that the expression of mucin genes by TGFBIp is mediated by the activation of ERK and AKT signaling.
CONCLUSION
Our findings demonstrate that the locally generated TGFBIp in the cornea may contribute to wound healing of CECs by enhancing the migration, adhesion, and proliferation of CECs. In addition, our results suggest that TGFBIp has a protective effect on ocular surfaces by inducing the expression of mucin genes in corneal and conjunctival epithelial cells. These data suggest that TGFBIp is a useful therapeutic target for patients with corneal wounds.
Keyword
MeSH Terms
-
Blotting, Western
Cell Adhesion
Cell Movement/drug effects
Cell Proliferation
Cells, Cultured
Conjunctiva/cytology
Cornea/*cytology
Epithelial Cells/*metabolism
Extracellular Matrix Proteins/*physiology
Gene Expression
Humans
Mucins/genetics/*metabolism
Signal Transduction
Transforming Growth Factor beta/*physiology
Wound Healing/*physiology
Extracellular Matrix Proteins
Mucins
Transforming Growth Factor beta
Figure
Reference
-
1. Knop E, Knop N, Millar T, Obata H, Sullivan DA. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci. 2011; 52:1938–1978.
Article2. Gipson IK, Argüeso P. Role of mucins in the function of the corneal and conjunctival epithelia. Int Rev Cytol. 2003; 231:1–49.
Article3. Dartt DA. Control of mucin production by ocular surface epithelial cells. Exp Eye Res. 2004; 78:173–185.
Article4. Spurr-Michaud S, Argüeso P, Gipson I. Assay of mucins in human tear fluid. Exp Eye Res. 2007; 84:939–950.
Article5. Carraway KL, Perez A, Idris N, Jepson S, Arango M, Komatsu M, et al. MUC4/sialomucin complex, the intramembrane ErbB2 ligand, in cancer and epithelia: to protect and to survive. Prog Nucleic Acid Res Mol Biol. 2002; 71:149–185.6. Argüeso P. Glycobiology of the ocular surface: mucins and lectins. Jpn J Ophthalmol. 2013; 57:150–155.
Article7. Gipson IK. Distribution of mucins at the ocular surface. Exp Eye Res. 2004; 78:379–388.
Article8. Argüeso P, Guzman-Aranguez A, Mantelli F, Cao Z, Ricciuto J, Panjwani N. Association of cell surface mucins with galectin-3 contributes to the ocular surface epithelial barrier. J Biol Chem. 2009; 284:23037–23045.
Article9. Jonckheere N, Van Seuningen I. The membrane-bound mucins: from cell signalling to transcriptional regulation and expression in epithelial cancers. Biochimie. 2010; 92:1–11.
Article10. McGuckin MA, Quin RJ, Ward BG. Progesterone stimulates production and secretion of MUC1 epithelial mucin in steroid-responsive breast cancer cell lines. Int J Oncol. 1998; 12:939–945.
Article11. Treon SP, Mollick JA, Urashima M, Teoh G, Chauhan D, Ogata A, et al. MUC-1 core protein is expressed on multiple myeloma cells and is induced by dexamethasone. Blood. 1999; 93:1287–1298.
Article12. Theodoropoulos G, Carraway KL. Molecular signaling in the regulation of mucins. J Cell Biochem. 2007; 102:1103–1116.
Article13. Hori Y, Spurr-Michaud S, Russo CL, Argüeso P, Gipson IK. Differential regulation of membrane-associated mucins in the human ocular surface epithelium. Invest Ophthalmol Vis Sci. 2004; 45:114–122.
Article14. Ma M, Zhang Z, Niu W, Zheng W, Kelimu J, Ke B. Fibroblast growth factor 10 upregulates the expression of mucins in rat conjunctival epithelial cells. Mol Vis. 2011; 17:2789–2797.15. Hori Y, Spurr-Michaud SJ, Russo CL, Argüeso P, Gipson IK. Effect of retinoic acid on gene expression in human conjunctival epithelium: secretory phospholipase A2 mediates retinoic acid induction of MUC16. Invest Ophthalmol Vis Sci. 2005; 46:4050–4061.
Article16. Seo KY, Chung SH, Lee JH, Park MY, Kim EK. Regulation of membrane-associated mucins in the human corneal epithelial cells by dexamethasone. Cornea. 2007; 26:709–714.
Article17. Li S, Bobek LA. Functional analysis of human MUC7 mucin gene 5′-flanking region in lung epithelial cells. Am J Respir Cell Mol Biol. 2006; 35:593–601.
Article18. Tei M, Moccia R, Gipson IK. Developmental expression of mucin genes ASGP (rMuc4) and rMuc5ac by the rat ocular surface epithelium. Invest Ophthalmol Vis Sci. 1999; 40:1944–1951.19. Thapa N, Lee BH, Kim IS. TGFBIp/betaig-h3 protein: a versatile matrix molecule induced by TGF-beta. Int J Biochem Cell Biol. 2007; 39:2183–2194.20. Gibson MA, Kumaratilake JS, Cleary EG. Immunohistochemical and ultrastructural localization of MP78/70 (betaig-h3) in extracellular matrix of developing and mature bovine tissues. J Histochem Cytochem. 1997; 45:1683–1696.
Article21. Escribano J, Hernando N, Ghosh S, Crabb J, Coca-Prados M. cDNA from human ocular ciliary epithelium homologous to beta ig-h3 is preferentially expressed as an extracellular protein in the corneal epithelium. J Cell Physiol. 1994; 160:511–521.
Article22. Billings PC, Herrick DJ, Kucich U, Engelsberg BN, Abrams WR, Macarak EJ, et al. Extracellular matrix and nuclear localization of beta ig-h3 in human bladder smooth muscle and fibroblast cells. J Cell Biochem. 2000; 79:261–273.
Article23. Karring H, Runager K, Valnickova Z, Thøgersen IB, Møller-Pedersen T, Klintworth GK, et al. Differential expression and processing of transforming growth factor beta induced protein (TGFBIp) in the normal human cornea during postnatal development and aging. Exp Eye Res. 2010; 90:57–62.
Article24. Kim JE, Kim EH, Han EH, Park RW, Park IH, Jun SH, et al. A TGF-beta-inducible cell adhesion molecule, betaig-h3, is downregulated in melorheostosis and involved in osteogenesis. J Cell Biochem. 2000; 77:169–178.
Article25. Bae JS, Lee SH, Kim JE, Choi JY, Park RW, Park JY, et al. Betaig-h3 supports keratinocyte adhesion, migration, and proliferation through alpha3beta1 integrin. Biochem Biophys Res Commun. 2002; 294:940–948.
Article26. Zhang Y, Wen G, Shao G, Wang C, Lin C, Fang H, et al. TGFBI deficiency predisposes mice to spontaneous tumor development. Cancer Res. 2009; 69:37–44.
Article27. Maeng YS, Aguilar B, Choi SI, Kim EK. Inhibition of TGFBIp expression reduces lymphangiogenesis and tumor metastasis. Oncogene. 2016; 35:196–205.
Article28. Park SW, Bae JS, Kim KS, Park SH, Lee BH, Choi JY, et al. Beta ig-h3 promotes renal proximal tubular epithelial cell adhesion, migration and proliferation through the interaction with alpha3beta1 integrin. Exp Mol Med. 2004; 36:211–219.
Article29. Kim YH, Kwon HJ, Kim DS. Matrix metalloproteinase 9 (MMP-9)-dependent processing of βig-h3 protein regulates cell migration, invasion, and adhesion. J Biol Chem. 2012; 287:38957–38969.
Article30. Fujiki K, Hotta Y, Nakayasu K, Yokoyama T, Takano T, Yamaguchi T, et al. A new L527R mutation of the betaIGH3 gene in patients with lattice corneal dystrophy with deep stromal opacities. Hum Genet. 1998; 103:286–289.
Article31. Streeten BW, Qi Y, Klintworth GK, Eagle RC Jr, Strauss JA, Bennett K. Immunolocalization of beta ig-h3 protein in 5q31-linked corneal dystrophies and normal corneas. Arch Ophthalmol. 1999; 117:67–75.
Article32. Runager K, Klintworth GK, Karring H, Enghild JJ. The insoluble TGFBIp fraction of the cornea is covalently linked via a disulfide bond to type XII collagen. Biochemistry. 2013; 52:2821–2827.
Article33. Araki-Sasaki K, Ohashi Y, Sasabe T, Hayashi K, Watanabe H, Tano Y, et al. An SV40-immortalized human corneal epithelial cell line and its characterization. Invest Ophthalmol Vis Sci. 1995; 36:614–621.34. Lee H, Kim EK, Kim JY, Yang YM, Shin DM, Kang KK, et al. DA-6034-induced mucin secretion via Ca2+-dependent pathways through P2Y receptor stimulation. Invest Ophthalmol Vis Sci. 2014; 55:6565–6574.
Article35. Maldonado BA, Furcht LT. Epidermal growth factor stimulates integrin-mediated cell migration of cultured human corneal epithelial cells on fibronectin and arginine-glycine-aspartic acid peptide. Invest Ophthalmol Vis Sci. 1995; 36:2120–2126.36. Maeng YS, Choi HJ, Kwon JY, Park YW, Choi KS, Min JK, et al. Endothelial progenitor cell homing: prominent role of the IGF2-IGF2R-PLCbeta2 axis. Blood. 2009; 113:233–243.
Article37. Situ H, Bobek LA. In vitro assessment of antifungal therapeutic potential of salivary histatin-5, two variants of histatin-5, and salivary mucin (MUC7) domain 1. Antimicrob Agents Chemother. 2000; 44:1485–1493.
Article38. Choi SI, Maeng YS, Kim TI, Lee Y, Kim YS, Kim EK. Lysosomal trafficking of TGFBIp via caveolae-mediated endocytosis. PLoS One. 2015; 10:e0119561.
Article39. Maeng YS, Choi YJ, Kim EK. TGFBIp regulates differentiation of EPC (CD133(+) C-kit(+) Lin(-) cells) to EC through activation of the Notch signaling pathway. Stem Cells. 2015; 33:2052–2062.
Article40. Huveneers S, Danen EH. Adhesion signaling-crosstalk between integrins, Src and Rho. J Cell Sci. 2009; 122(Pt 8):1059–1069.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Expression and Distribution of MUC1 in Human Corneal Epithelium
- Effect of Epidermal Growth Factor on the Wound Healing after Excimer Laser Photokeratectomy in Rabbits
- Effects of 0.1% Dexamethasone on Experimental corneal Epithelial Healing Following Alkali Wounds
- Comparison of Wound Healing and Inflammation Depending on the ablation Methods in Rabbits
- Suppressed Secretion of The Extracellular Matrix of The Cultured Corneal Epithelial Cell following Inoculation of HSV-1