Clin Exp Vaccine Res.
2016 Jul;5(2):159-168. 10.7774/cevr.2016.5.2.159.
Safety and immunogenicity of recombinant rabies virus (ERAGS) in mice and raccoon dogs
- Affiliations
-
- 1Viral Disease Division, Animal and Plant Quarantine Agency, MAFRA, Gimcheon, Korea. yangdk@korea.kr
- 2College of Veterinary Medicine, Kangwon National University, Chuncheon, Korea.
- 3Jeonnam Wildlife Management Center, Suncheon, Korea.
- KMID: 2421563
- DOI: http://doi.org/10.7774/cevr.2016.5.2.159
Abstract
- PURPOSE
The development of a genetically modified live rabies vaccine applicable to wild raccoon dogs is necessary for the eradication of rabies in Korea. Thus, we constructed a recombinant rabies virus (RABV) called the ERAGS strain, using a reverse genetic system and evaluated its safety and efficacy in mice and its safety and immunogenicity in raccoon dogs.
MATERIALS AND METHODS
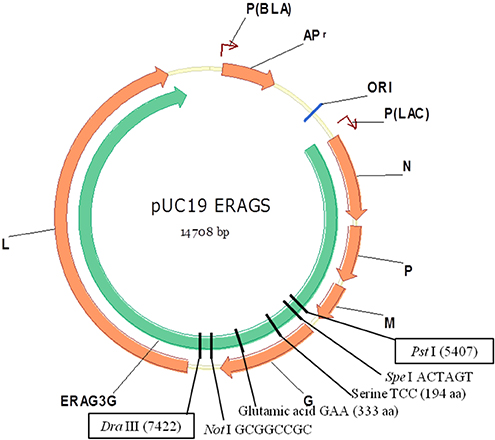
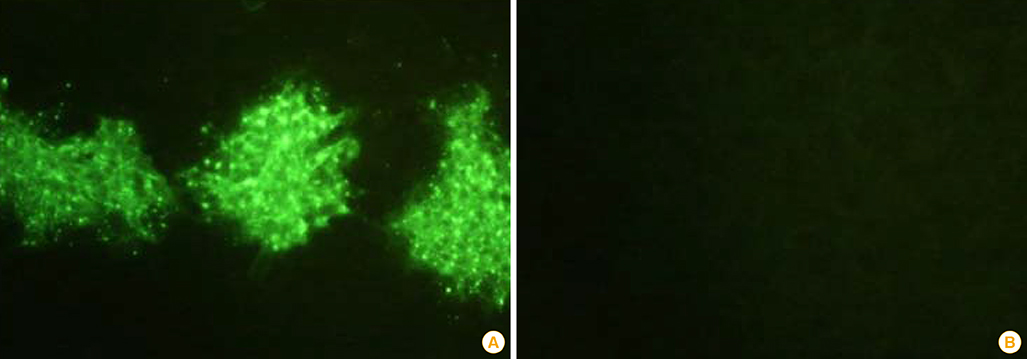
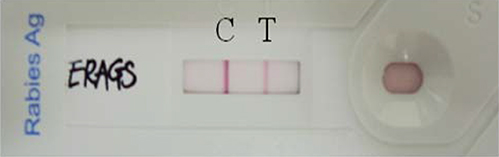
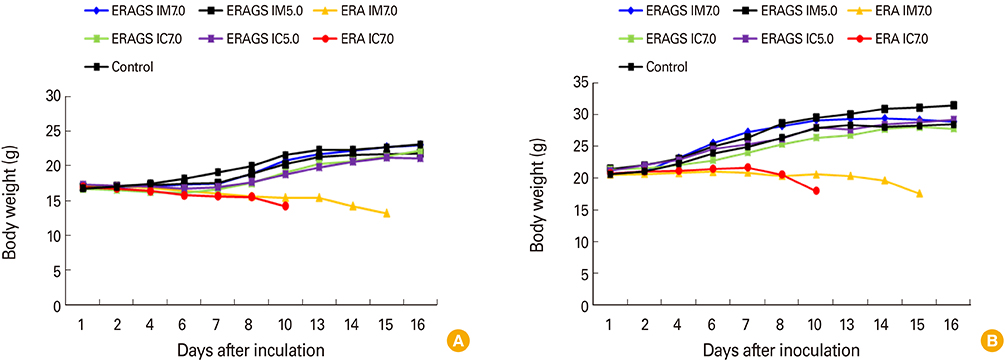
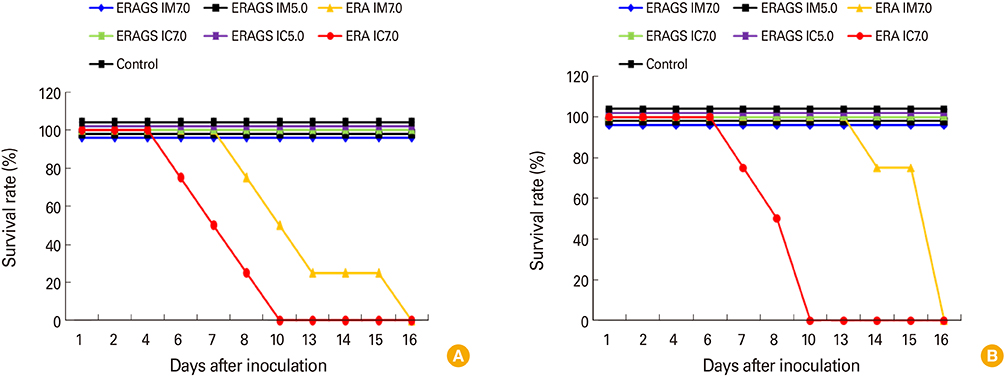
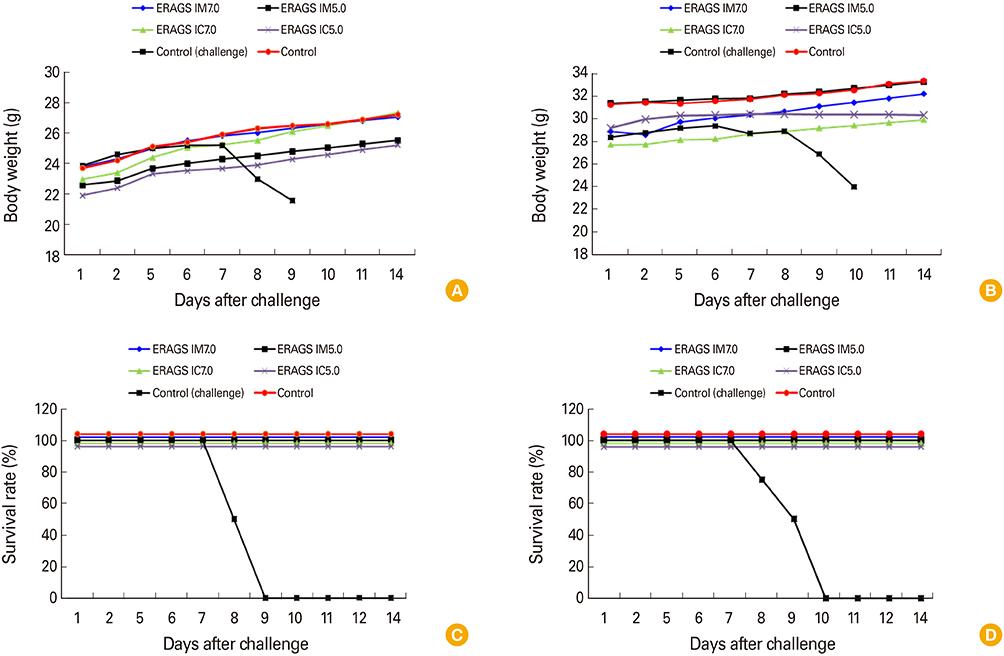
ERAGS, which has Asn194Ser and Arg333Glu substitutions in the glycoprotein, was constructed using site-directed mutagenesis. Mice were inoculated with the ERAGS strain (either 10(5.0) or 10(7.0) FAID(50)/mL) via intramuscular (IM) or intracranial injections and then challenged with a virulent RABV. Raccoon dogs were administered the ERAGS strain (10(8.0) FAID(50)/mL) either orally or via the IM route and the immunogenicity of the strain was evaluated using fluorescent antibody virus neutralization tests.
RESULTS
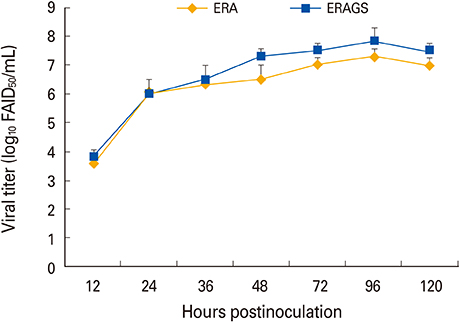
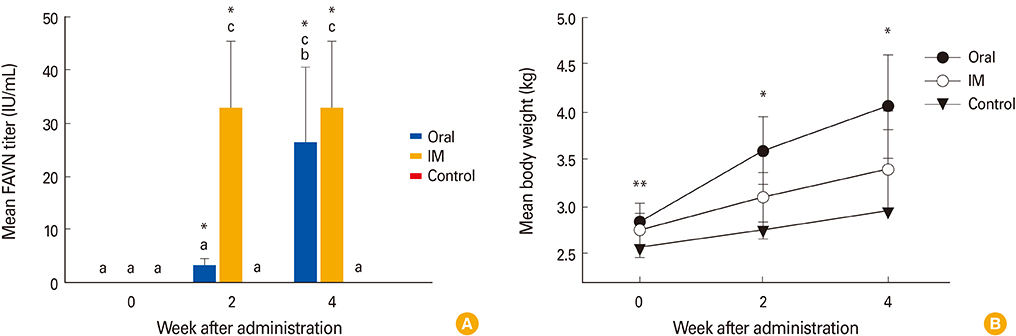
The ERAGS strain inoculated into murine neuroblastoma cells reached 10(7.8) FAID(50)/mL at 96-hour post-inoculation. The virus was not pathogenic and induced complete protection from virulent RABV in immunized 4- and 6-week-old mice. Korean raccoon dogs immunized with the ERAGS strain via IM or oral route were also safe from the virus and developed high titer levels (26.4-32.8 IU/mL) of virus-neutralizing antibody (VNA) at 4 weeks post-inoculation.
CONCLUSION
The ERAGS RABV strain was effectively protective against rabies in mice and produced a high VNA titer in raccoon dogs.
Keyword
MeSH Terms
Figure
Cited by 3 articles
-
Establishment of multiplex RT-PCR for differentiation between rabies virus with and that without mutation at position 333 of glycoprotein
Dong-Kun Yang, Ha-Hyun Kim, Siu Lee, Jae-Young Yoo
J Vet Sci. 2020;21(2):e22. doi: 10.4142/jvs.2020.21.e22.A genetically modified rabies vaccine (ERAGS) induces protective immunity in dogs and cattle
Dong-Kun Yang, Ha-Hyun Kim, Seung Heon Lee, Woong-Ho Jeong, Dongseop Tark, In-Soo Cho
Clin Exp Vaccine Res. 2017;6(2):128-134. doi: 10.7774/cevr.2017.6.2.128.Establishment of multiplex RT-PCR for differentiation between rabies virus with and that without mutation at position 333 of glycoprotein
Dong-Kun Yang, Ha-Hyun Kim, Siu Lee, Jae-Young Yoo
J Vet Sci. 2020;21(2):. doi: 10.4142/jvs.2020.21.e22.
Reference
-
1. Seneschall C, Luna-Farro M. Controlling rabies through a multidisciplinary, public health system in Trujillo, La Libertad, Peru. Pathog Glob Health. 2013; 107:361–366.
Article2. Hyun BH, Lee KK, Kim IJ, et al. Molecular epidemiology of rabies virus isolates from South Korea. Virus Res. 2005; 114:113–125.
Article3. Mahl P, Cliquet F, Guiot AL, et al. Twenty year experience of the oral rabies vaccine SAG2 in wildlife: a global review. Vet Res. 2014; 45:77.
Article4. Brown LJ, Rosatte RC, Fehlner-Gardiner C, et al. Oral vaccination and protection of red foxes (Vulpes vulpes) against rabies using ONRAB, an adenovirus-rabies recombinant vaccine. Vaccine. 2014; 32:984–989.
Article5. Fehlner-Gardiner C, Rudd R, Donovan D, Slate D, Kempf L, Badcock J. Comparing ONRAB(R) AND RABORAL V-RG(R) oral rabies vaccine field performance in raccoons and striped skunks, New Brunswick, Canada, and Maine, USA. J Wildl Dis. 2012; 48:157–167.
Article6. World Health Organization. WHO Expert Consultation on Rabies. World Health Organ Tech Rep Ser. 2005; 931:1–88.7. Faber M, Dietzschold B, Li J. Immunogenicity and safety of recombinant rabies viruses used for oral vaccination of stray dogs and wildlife. Zoonoses Public Health. 2009; 56:262–269.
Article8. Lawson KF, Chiu H, Crosgrey SJ, Matson M, Casey GA, Campbell JB. Duration of immunity in foxes vaccinated orally with ERA vaccine in a bait. Can J Vet Res. 1997; 61:39–42.9. Yang DK, Go TO, Nam YH, et al. Antibody response in Korean raccoon dogs inoculated with inactivated rabies vaccines. J Bacteriol Virol. 2012; 42:242–246.
Article10. Albertini AA, Schoehn G, Weissenhorn W, Ruigrok RW. Structural aspects of rabies virus replication. Cell Mol Life Sci. 2008; 65:282–294.
Article11. Raux H, Flamand A, Blondel D. Interaction of the rabies virus P protein with the LC8 dynein light chain. J Virol. 2000; 74:10212–10216.
Article12. Dietzschold B, Wunner WH, Wiktor TJ, et al. Characterization of an antigenic determinant of the glycoprotein that correlates with pathogenicity of rabies virus. Proc Natl Acad Sci U S A. 1983; 80:70–74.
Article13. Seif I, Coulon P, Rollin PE, Flamand A. Rabies virulence: effect on pathogenicity and sequence characterization of rabies virus mutations affecting antigenic site III of the glycoprotein. J Virol. 1985; 53:926–934.
Article14. Tuffereau C, Leblois H, Benejean J, Coulon P, Lafay F, Flamand A. Arginine or lysine in position 333 of ERA and CVS glycoprotein is necessary for rabies virulence in adult mice. Virology. 1989; 172:206–212.
Article15. Faber M, Faber ML, Papaneri A, et al. A single amino acid change in rabies virus glycoprotein increases virus spread and enhances virus pathogenicity. J Virol. 2005; 79:14141–14148.
Article16. Ito N, Takayama-Ito M, Yamada K, Hosokawa J, Sugiyama M, Minamoto N. Improved recovery of rabies virus from cloned cDNA using a vaccinia virus-free reverse genetics system. Microbiol Immunol. 2003; 47:613–617.
Article17. Cliquet F, Aubert M, Sagne L. Development of a fluorescent antibody virus neutralisation test (FAVN test) for the quantitation of rabies-neutralising antibody. J Immunol Methods. 1998; 212:79–87.
Article18. Cliquet F, Gurbuxani JP, Pradhan HK, et al. The safety and efficacy of the oral rabies vaccine SAG2 in Indian stray dogs. Vaccine. 2007; 25:3409–3418.
Article19. Cliquet F, Robardet E, Must K, et al. Eliminating rabies in Estonia. PLoS Negl Trop Dis. 2012; 6:e1535.
Article20. Lafay F, Benejean J, Tuffereau C, Flamand A, Coulon P. Vaccination against rabies: construction and characterization of SAG2, a double avirulent derivative of SADBern. Vaccine. 1994; 12:317–320.
Article21. Takayama-Ito M, Inoue K, Shoji Y, et al. A highly attenuated rabies virus HEP-Flury strain reverts to virulent by single amino acid substitution to arginine at position 333 in glycoprotein. Virus Res. 2006; 119:208–215.
Article22. Dietzschold ML, Faber M, Mattis JA, Pak KY, Schnell MJ, Dietzschold B. In vitro growth and stability of recombinant rabies viruses designed for vaccination of wildlife. Vaccine. 2004; 23:518–524.
Article23. Rupprecht CE, Blass L, Smith K, et al. Human infection due to recombinant vaccinia-rabies glycoprotein virus. N Engl J Med. 2001; 345:582–586.
Article24. Shuai L, Feng N, Wang X, et al. Genetically modified rabies virus ERA strain is safe and induces long-lasting protective immune response in dogs after oral vaccination. Antiviral Res. 2015; 121:9–15.
Article25. Yang DK, Nakagawa K, Ito N, et al. A single immunization with recombinant rabies virus (ERAG3G) confers complete protection against rabies in mice. Clin Exp Vaccine Res. 2014; 3:176–184.
Article26. Nakagawa K, Ito N, Masatani T, et al. Generation of a live rabies vaccine strain attenuated by multiple mutations and evaluation of its safety and efficacy. Vaccine. 2012; 30:3610–3617.
Article27. Bankovskiy D, Safonov G, Kurilchuk Y. Immunogenicity of the ERA G 333 rabies virus strain in foxes and raccoon dogs. Dev Biol (Basel). 2008; 131:461–466.28. Mebatsion T. Extensive attenuation of rabies virus by simultaneously modifying the dynein light chain binding site in the P protein and replacing Arg333 in the G protein. J Virol. 2001; 75:11496–11502.
Article29. Franka R, Wu X, Jackson FR, et al. Rabies virus pathogenesis in relationship to intervention with inactivated and attenuated rabies vaccines. Vaccine. 2009; 27:7149–7155.
Article30. Orciari LA, Niezgoda M, Hanlon CA, et al. Rapid clearance of SAG-2 rabies virus from dogs after oral vaccination. Vaccine. 2001; 19:4511–4518.
Article31. Ogra PL, Faden H, Welliver RC. Vaccination strategies for mucosal immune responses. Clin Microbiol Rev. 2001; 14:430–445.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Safety and Immunogenicity of a Recombinant Rabies Virus Strain (ERAG3G) in Korean Raccoon Dogs
- Application of recombinant adenoviruses expressing glycoprotein or nucleoprotein of rabies virus to Korean raccoon dogs
- Immunogenicity of an inactivated rabies vaccine for animals derived from the recombinant ERAGS strain
- A genetically modified rabies vaccine (ERAGS) induces protective immunity in dogs and cattle
- Antibody Response in Korean Raccoon Dogs Inoculated with Inactivated Rabies Vaccines