Clin Exp Vaccine Res.
2015 Jul;4(2):189-194. 10.7774/cevr.2015.4.2.189.
Application of recombinant adenoviruses expressing glycoprotein or nucleoprotein of rabies virus to Korean raccoon dogs
- Affiliations
-
- 1College of Veterinary Medicine, Kangwon National University, Chuncheon, Korea.
- 2Viral Disease Division, Animal and Plant Quarantine Agency, MAFRA, Anyang, Korea. yangdk@korea.kr
- 3Wild Life Center, Gyeonggi-do Veterinary Service Laboratory, Pyeongtack, Korea.
- KMID: 1965404
- DOI: http://doi.org/10.7774/cevr.2015.4.2.189
Abstract
- PURPOSE
A new rabies vaccine for animals, including raccoon dogs, in Korea is needed to eradicate rabies infection. In this study, we constructed two recombinant adenoviruses expressing the glycoprotein or nucleoprotein of the rabies virus (RABV). We then investigated the safety and immunogenicity of these strains in raccoon dogs, depending on inoculation route.
MATERIALS AND METHODS
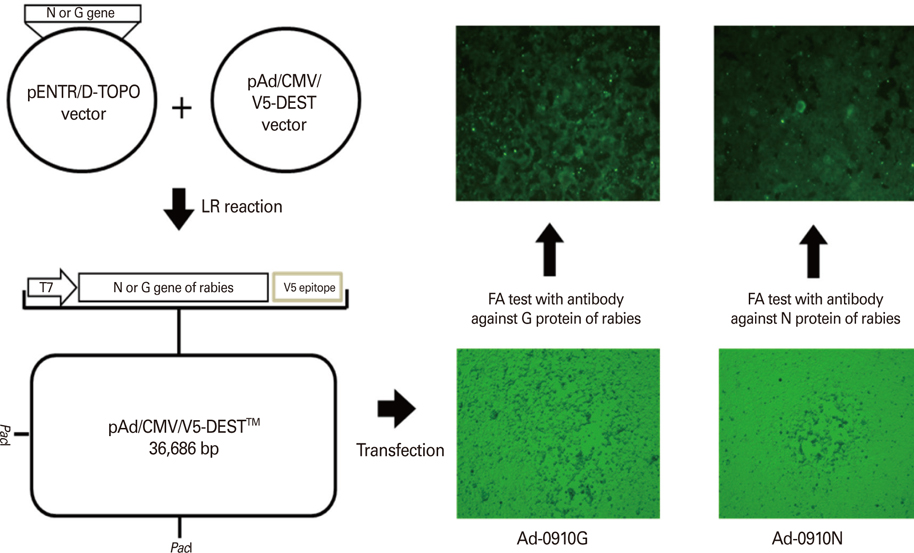
Recombinant adenoviruses expressing the glycoprotein (Ad-0910G) or nucleoprotein (Ad-0910N) of rabies were constructed in 293A cells using an adenoviral system. One-year-old raccoon dogs underwent intramuscular (IM) inoculation or oral administration of the recombinant Ad-0910G and Ad-0910N. Clinical symptoms were observed and virus-neutralizing antibodies (VNA) against RABV were measured at 0, 2, 4, and 6 weeks after the immunization. Raccoons were considered positive if VNA titers were > or = 0.1 IU/mL.
RESULTS
Raccoon dogs inoculated with the combined Ad-0910G and Ad-0910N virus via the IM route did not exhibit any clinical sign of rabies during the observation period. All raccoon dogs (n = 7) immunized IM had high VNA titers, ranging from 0.17 to 41.6 IU/mL at 2 weeks after inoculation, but 70% (7/10) of raccoon dogs administered viruses via the oral route responded by 6 weeks after administration against RABV.
CONCLUSION
Raccoon dogs inoculated with Ad-0910G and Ad-0910N viruses showed no adverse effects. Immunization with the combined Ad-0910G and Ad-0910N strains may play an important role in inducing VNA against RABV in raccoon dogs.
Keyword
MeSH Terms
Figure
Reference
-
1. Yang DK, Kim SY, Oh YI, et al. Epidemiological characteristics of rabies in South Korea from January 2004 to March 2011. J Bacteriol Virol. 2011; 41:165–171.
Article2. Park JS, Kim CK, Kim SY, Ju YR. Molecular characterization of KGH, the first human isolate of rabies virus in Korea. Virus Genes. 2013; 46:231–241.
Article3. Joo YS, Lee JH, Lee KK, Bang HA, Lee WC. Retrospective study of extensive vaccination programs for canine rabies control and public health in Korea. Jpn J Infect Dis. 2011; 64:513–515.4. Oem JK, Kim SH, Kim YH, Lee MH, Lee KK. Reemergence of rabies in the southern Han River region, Korea. J Wildl Dis. 2014; 50:681–688.
Article5. Sterner RT, Meltzer MI, Shwiff SA, Slate D. Tactics and economics of wildlife oral rabies vaccination, Canada and the United States. Emerg Infect Dis. 2009; 15:1176–1184.
Article6. Cliquet F, Aubert M. Elimination of terrestrial rabies in Western European countries. Dev Biol (Basel). 2004; 119:185–204.7. Cheong Y, Kim B, Lee KJ, et al. Strategic model of national rabies control in Korea. Clin Exp Vaccine Res. 2014; 3:78–90.
Article8. Orciari LA, Niezgoda M, Hanlon CA, et al. Rapid clearance of SAG-2 rabies virus from dogs after oral vaccination. Vaccine. 2001; 19:4511–4518.
Article9. Cliquet F, Gurbuxani JP, Pradhan HK, et al. The safety and efficacy of the oral rabies vaccine SAG2 in Indian stray dogs. Vaccine. 2007; 25:3409–3418.
Article10. Rupprecht CE, Blass L, Smith K, et al. Human infection due to recombinant vaccinia-rabies glycoprotein virus. N Engl J Med. 2001; 345:582–586.
Article11. Rupprecht CE, Hanlon CA, Slate D. Oral vaccination of wildlife against rabies: opportunities and challenges in prevention and control. Dev Biol (Basel). 2004; 119:173–184.12. Li J, Faber M, Papaneri A, et al. A single immunization with a recombinant canine adenovirus expressing the rabies virus G protein confers protective immunity against rabies in mice. Virology. 2006; 356:147–154.
Article13. Fehlner-Gardiner C, Rudd R, Donovan D, Slate D, Kempf L, Badcock J. Comparing ONRAB(R) AND RABORAL V-RG(R) oral rabies vaccine field performance in raccoons and striped skunks, New Brunswick, Canada, and Maine, USA. J Wildl Dis. 2012; 48:157–167.
Article14. Brown LJ, Rosatte RC, Fehlner-Gardiner C, et al. Oral vaccination and protection of red foxes (Vulpes vulpes) against rabies using ONRAB, an adenovirus-rabies recombinant vaccine. Vaccine. 2014; 32:984–989.
Article15. McCoy K, Tatsis N, Korioth-Schmitz B, et al. Effect of preexisting immunity to adenovirus human serotype 5 antigens on the immune responses of nonhuman primates to vaccine regimens based on human- or chimpanzee-derived adenovirus vectors. J Virol. 2007; 81:6594–6604.
Article16. Cliquet F, Aubert M, Sagne L. Development of a fluorescent antibody virus neutralisation test (FAVN test) for the quantitation of rabies-neutralising antibody. J Immunol Methods. 1998; 212:79–87.
Article17. Kim YR. Prophylaxis of human hydrophobia in South Korea. Infect Chemother. 2014; 46:143–148.
Article18. Park JH, Lee CH, Won YK, Chin BS, Shin HS, Kim JY. Rabies post-exposure prophylaxis of overseas travelers in the international travel clinic of the national medical center from 2006 to 2012, Korea. Infect Chemother. 2014; 46:13–20.
Article19. Franka R, Smith TG, Dyer JL, Wu X, Niezgoda M, Rupprecht CE. Current and future tools for global canine rabies elimination. Antiviral Res. 2013; 100:220–225.
Article20. Macfarlan RI, Dietzschold B, Koprowski H. Stimulation of cytotoxic T-lymphocyte responses by rabies virus glycoprotein and identification of an immunodominant domain. Mol Immunol. 1986; 23:733–741.
Article21. Sumner JW, Fekadu M, Shaddock JH, Esposito JJ, Bellini WJ. Protection of mice with vaccinia virus recombinants that express the rabies nucleoprotein. Virology. 1991; 183:703–710.
Article22. Zhang J. Advances and future challenges in recombinant adenoviral vectored H5N1 influenza vaccines. Viruses. 2012; 4:2711–2735.
Article23. Deal C, Pekosz A, Ketner G. Prospects for oral replicating adenovirus-vectored vaccines. Vaccine. 2013; 31:3236–3243.
Article24. Richardson JS, Abou MC, Tran KN, Kumar A, Sahai BM, Kobinger GP. Impact of systemic or mucosal immunity to adenovirus on Ad-based Ebola virus vaccine efficacy in guinea pigs. J Infect Dis. 2011; 204:Suppl 3. S1032–S1042.
Article25. Wang Y, Xiang Z, Pasquini S, Ertl HC. The use of an E1-deleted, replication-defective adenovirus recombinant expressing the rabies virus glycoprotein for early vaccination of mice against rabies virus. J Virol. 1997; 71:3677–3683.
Article26. Slate D, Chipman RB, Algeo TP, et al. Safety and immunogenicity of Ontario Rabies Vaccine Bait (ONRAB) in the first us field trial in raccoons (Procyon lotor). J Wildl Dis. 2014; 50:582–595.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Safety and Immunogenicity of a Recombinant Rabies Virus Strain (ERAG3G) in Korean Raccoon Dogs
- Outbreaks and Control of Animal Rabies in Korea
- Safety and immunogenicity of recombinant rabies virus (ERAGS) in mice and raccoon dogs
- Indirect ELISA for the Detection of Rabies Virus Antibodies in Dog Sera
- Detection of viral infections in wild Korean raccoon dogs (Nyctereutes procyonoides koreensis)