Clin Exp Vaccine Res.
2017 Jul;6(2):128-134. 10.7774/cevr.2017.6.2.128.
A genetically modified rabies vaccine (ERAGS) induces protective immunity in dogs and cattle
- Affiliations
-
- 1Viral Disease Division, Animal and Plant Quarantine Agency, MAFRA, Gimcheon, Korea. yangdk@korea.kr
- 2Kangwondo Veterinary Service Laboratory, Pyeongchang, Korea.
- 3Korea Zoonosis Research Institute, Chonbuk National University, Iksan, Korea.
- KMID: 2391573
- DOI: http://doi.org/10.7774/cevr.2017.6.2.128
Abstract
- PURPOSE
The current live attenuated rabies vaccine must be replaced with a safer vaccine based on the ERAGS strain to prevent rabies in South Korea. We evaluated the safety and immunogenicity of a new strain in dogs and cattle.
MATERIALS AND METHODS
The ERAGS strain, featuring two mutations altering two amino acids in a glycoprotein of rabies virus, was propagated in NG108-15 cells. We lyophilized the virus in the presence of two different stabilizers to evaluate the utilities of such preparations as novel rabies vaccines for animals. To explore safety and immunogenicity, dogs and cattle were inoculated with the vaccine at various doses via different routes and observed daily for 8 weeks post-inoculation (WPI). Immunogenicity was evaluated using a fluorescent antibody virus neutralization test or enzyme-linked immunosorbent assay.
RESULTS
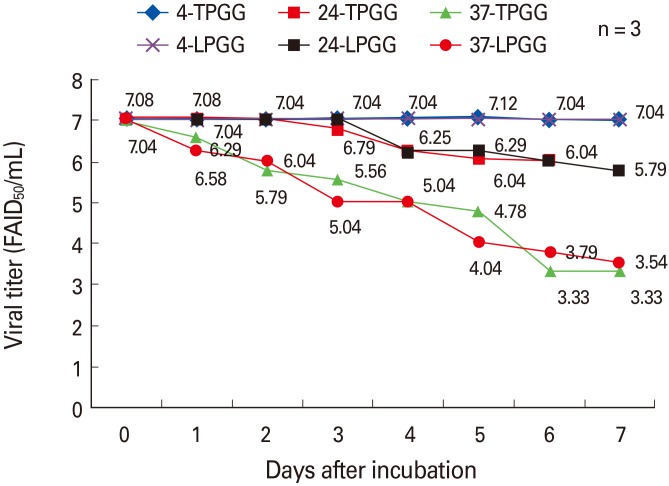
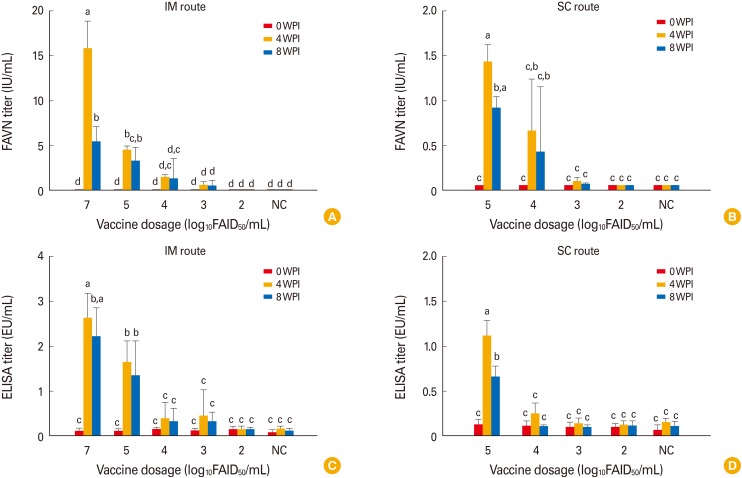
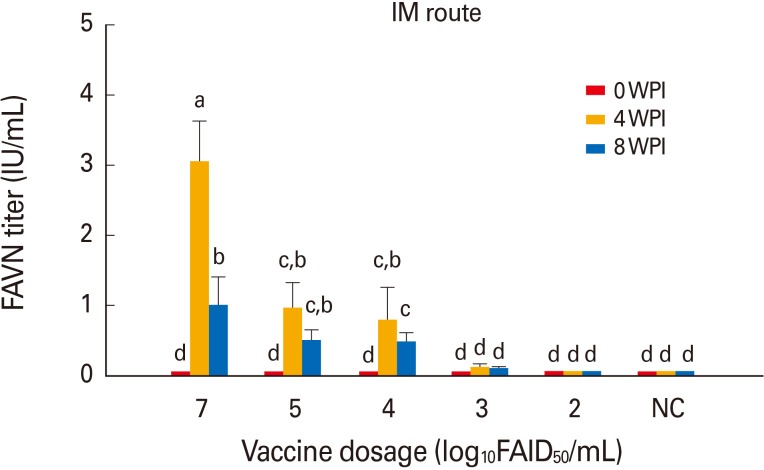
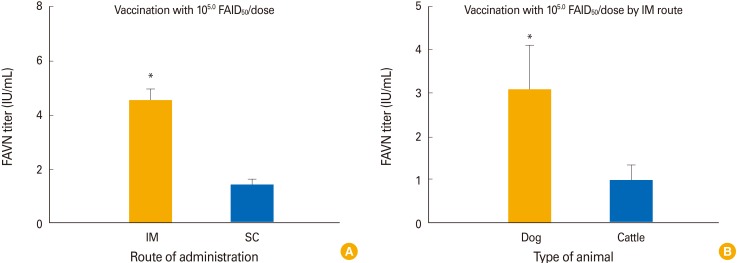
The two different stabilizers did not differ greatly in terms of maintenance of virus viability in accelerated stability testing. No clinical signs of rabies developed in dogs or cattle inoculated with the vaccines (10(7.0) FAIDâ‚…â‚€/mL). Dogs and cattle inoculated intramuscularly with 10(5.0) FAIDâ‚…â‚€/mL exhibited virus neutralization assay titers of 4.6 IU/mL and 1.5 to 0.87 IU/mL at 4 WPI, respectively. All control animals remained rabies virus-seronegative throughout, confirming that no contact transmission occurred between vaccinated and control animals.
CONCLUSION
Our findings indicate that the new rabies vaccine is safe and immunogenic in dogs and cattle.
MeSH Terms
Figure
Cited by 1 articles
-
Strategies to maintain Korea's animal rabies non-occurrence status
Dong-Kun Yang, Ha-Hyun Kim, In-Soo Cho
Clin Exp Vaccine Res. 2018;7(2):87-92. doi: 10.7774/cevr.2018.7.2.87.
Reference
-
1. Kim CH, Lee CG, Yoon HC, et al. Rabies, an emerging disease in Korea. J Vet Med B Infect Dis Vet Public Health. 2006; 53:111–115. PMID: 16629721.
Article2. Cheong Y, Kim B, Lee KJ, et al. Strategic model of national rabies control in Korea. Clin Exp Vaccine Res. 2014; 3:78–90. PMID: 24427765.
Article3. Tenzin , Dhand NK, Gyeltshen T, et al. Dog bites in humans and estimating human rabies mortality in rabies endemic areas of Bhutan. PLoS Negl Trop Dis. 2011; 5:e1391. PMID: 22132247.
Article4. Sudarshan MK, Madhusudana SN, Mahendra BJ, et al. Assessing the burden of human rabies in India: results of a national multi-center epidemiological survey. Int J Infect Dis. 2007; 11:29–35. PMID: 16678463.
Article5. Nakagawa K, Ito N, Masatani T, et al. Generation of a live rabies vaccine strain attenuated by multiple mutations and evaluation of its safety and efficacy. Vaccine. 2012; 30:3610–3617. PMID: 22464967.
Article6. Guo L, Feng N, Yang S, et al. Reverse genetic system for rabies virus vaccine Evelyn-Rokitnicki-Abelseth strain. Wei Sheng Wu Xue Bao. 2009; 49:949–954. PMID: 19873761.7. Yang DK, Kim HH, Jo HY, Kim HW, Choi SS, Cho IS. Safety and immunogenicity of a recombinant rabies virus strain (ERAG3G) in Korean raccoon dogs. J Bacteriol Virol. 2015; 45:250–255.
Article8. Faber M, Faber ML, Papaneri A, et al. A single amino acid change in rabies virus glycoprotein increases virus spread and enhances virus pathogenicity. J Virol. 2005; 79:14141–14148. PMID: 16254349.
Article9. Mebatsion T. Extensive attenuation of rabies virus by simultaneously modifying the dynein light chain binding site in the P protein and replacing Arg333 in the G protein. J Virol. 2001; 75:11496–11502. PMID: 11689631.
Article10. Wang Y, Tian Q, Xu X, et al. Recombinant rabies virus expressing IFNalpha1 enhanced immune responses resulting in its attenuation and stronger immunogenicity. Virology. 2014; 468-470:621–630. PMID: 25310498.11. Wen Y, Wang H, Wu H, et al. Rabies virus expressing dendritic cell-activating molecules enhances the innate and adaptive immune response to vaccination. J Virol. 2011; 85:1634–1644. PMID: 21106736.
Article12. Yang DK, Kim HH, Choi SS, et al. Safety and immunogenicity of recombinant rabies virus (ERAGS) in mice and raccoon dogs. Clin Exp Vaccine Res. 2016; 5:159–168. PMID: 27489806.
Article13. Reed LJ, Muench H. Simple method of estimating fifty per cent endpoints. Am J Hyg. 1938; 27:493–497.14. Kang MS, Jang H, Kim MC, et al. Development of a stabilizer for lyophilization of an attenuated duck viral hepatitis vaccine. Poult Sci. 2010; 89:1167–1170. PMID: 20460663.
Article15. Cliquet F, Aubert M, Sagne L. Development of a fluorescent antibody virus neutralisation test (FAVN test) for the quantitation of rabies-neutralising antibody. J Immunol Methods. 1998; 212:79–87. PMID: 9671155.
Article16. Habib S, Khan MA, Younus H. Thermal destabilization of stem bromelain by trehalose. Protein J. 2007; 26:117–124. PMID: 17203393.
Article17. Joo YS, Lee JH, Lee KK, Bang HA, Lee WC. Retrospective study of extensive vaccination programs for canine rabies control and public health in Korea. Jpn J Infect Dis. 2011; 64:513–515. PMID: 22116332.18. Shuai L, Feng N, Wang X, et al. Genetically modified rabies virus ERA strain is safe and induces long-lasting protective immune response in dogs after oral vaccination. Antiviral Res. 2015; 121:9–15. PMID: 26093157.
Article19. Kissmann J, Ausar SF, Rudolph A, et al. Stabilization of measles virus for vaccine formulation. Hum Vaccin. 2008; 4:350–359. PMID: 18382143.
Article20. Cliquet F, Gurbuxani JP, Pradhan HK, et al. The safety and efficacy of the oral rabies vaccine SAG2 in Indian stray dogs. Vaccine. 2007; 25:3409–3418. PMID: 17224221.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunogenicity of an inactivated rabies vaccine for animals derived from the recombinant ERAGS strain
- Safety and immunogenicity of recombinant rabies virus (ERAGS) in mice and raccoon dogs
- Outbreaks and Control of Animal Rabies in Korea
- Antibody Response in Korean Raccoon Dogs Inoculated with Inactivated Rabies Vaccines
- Antibody Response in Cattle and Guinea Pigs Inoculated with Rabies Vaccines