Clin Exp Vaccine Res.
2016 Jul;5(2):132-137. 10.7774/cevr.2016.5.2.132.
An oral Aujeszky's disease vaccine (YS-400) induces neutralizing antibody in pigs
- Affiliations
-
- 1Viral Disease Division, Animal and Plant Quarantine Agency, MAFRA, Gimcheon, Korea. yangdk@korea.kr
- KMID: 2421560
- DOI: http://doi.org/10.7774/cevr.2016.5.2.132
Abstract
- PURPOSE
Aujeszky's disease (AD) is an economically important disease affecting both wild and domestic pigs of the species Sus scrofa. A previous study yielded serological evidence of AD in Korean wild boars, which could spread AD to other animals. A new Aujeszky's disease virus (ADV) bait vaccine is required to prevent AD outbreaks in swine. In the present study, we investigated the safety and immunogenicity of a gE-deleted marker vaccine, strain YS-400, in young domestic pigs.
MATERIALS AND METHODS
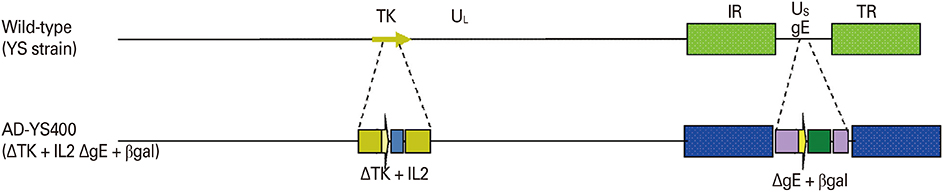
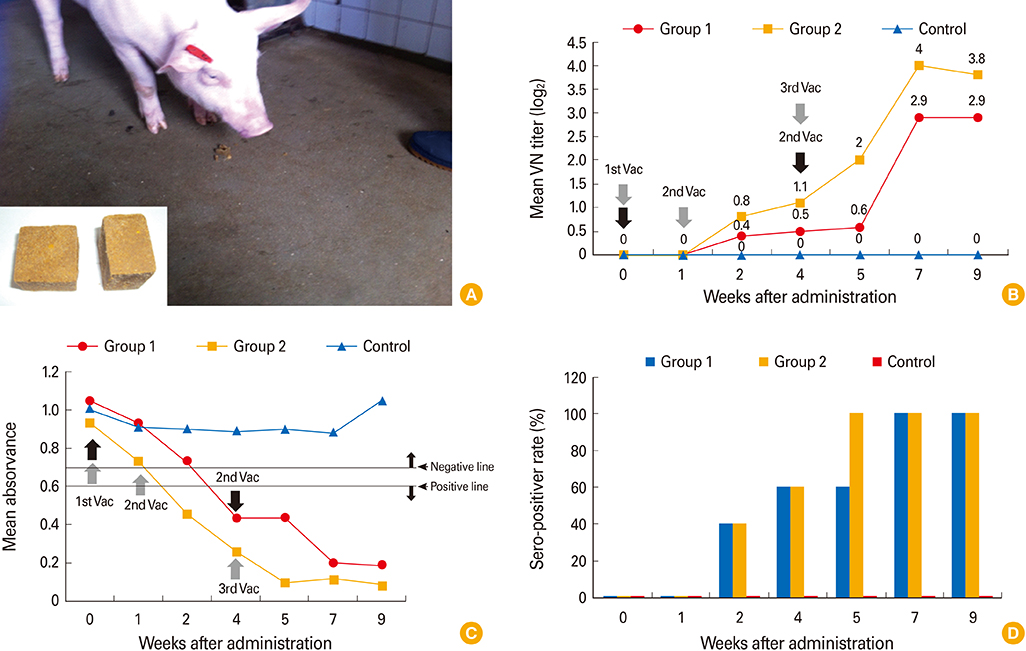
The YS-400 strain was propagated in Vero cells, and the trial ADV bait vaccine (a vaccine blister in a matrix including an attractant) was prepared. Pigs were orally immunized with the vaccine (2 mL, 10(7.5) TCID(50)/mL) delivered using a syringe or in the bait vaccine. The animals were observed for 9 weeks after vaccination, and immunogenicity was assessed using a virus neutralization (VN) test and enzyme linked immunosorbent assay.
RESULTS
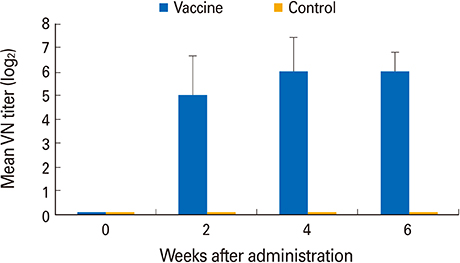
The YS-400 strain was non-pathogenic to pigs when given orally and induced high VN titers (1:32-1:128) 6 weeks post-administration. Of the pigs given the ADV bait vaccine twice or three times, 40% were seropositive by 2 weeks, and 100% were seropositive by 7 weeks after the first dose. Pigs that consumed the AD bait vaccine three times developed VN titers that were slightly higher than those of pigs given the vaccine twice.
CONCLUSION
Domestic pigs given the trial ADV bait vaccine exhibited no adverse effects and developed high VN titers against ADV, indicating that the YS-400 strain is safe and can prevent ADV infection in domestic pigs.
Keyword
MeSH Terms
Figure
Reference
-
1. Mettenleiter TC. Aujeszky's disease (pseudorabies) virus: the virus and molecular pathogenesis: state of the art, June 1999. Vet Res. 2000; 31:99–115.
Article2. Ferrari M, Gualandi GL, Corradi A, et al. Experimental infection of pigs with a thymidine kinase negative strain of pseudorabies virus. Comp Immunol Microbiol Infect Dis. 1998; 21:291–303.
Article3. Muller T, Batza HJ, Schluter H, Conraths FJ, Mettenleiter TC. Eradication of Aujeszky's disease in Germany. J Vet Med B Infect Dis Vet Public Health. 2003; 50:207–213.
Article4. Muller T, Hahn EC, Tottewitz F, et al. Pseudorabies virus in wild swine: a global perspective. Arch Virol. 2011; 156:1691–1705.
Article5. Wang CH, Yuan J, Qin HY, et al. A novel gE-deleted pseudorabies virus (PRV) provides rapid and complete protection from lethal challenge with the PRV variant emerging in Bartha-K61-vaccinated swine population in China. Vaccine. 2014; 32:3379–3385.
Article6. Yang DK, Nah JJ, Kim HH, et al. Seroepidemiological aurvey of Aujeszky's sisease cirus in wild boar (Sus scrofa) and raccoon dogs (Nyctereutes procyonoides koreensis) in Korea. J Bacteriol Virol. 2014; 44:336–341.
Article7. Pannwitz G, Freuling C, Denzin N, et al. A long-term serological survey on Aujeszky's disease virus infections in wild boar in East Germany. Epidemiol Infect. 2012; 140:348–358.
Article8. Boadella M, Gortazar C, Vicente J, Ruiz-Fons F. Wild boar: an increasing concern for Aujeszky's disease control in pigs? BMC Vet Res. 2012; 8:7.
Article9. Sedlak K, Bartova E, Machova J. Antibodies to selected viral disease agents in wild boars from the Czech Republic. J Wildl Dis. 2008; 44:777–780.
Article10. Mainguy J, Fehlner-Gardiner C, Slate D, Rudd RJ. Oral rabies vaccination in raccoons: comparison of ONRAB(R) and RABORAL V-RG(R) vaccine-bait field performance in Quebec, Canada and Vermont, USA. J Wildl Dis. 2013; 49:190–193.
Article11. Kaden V, Lange E, Fischer U, Strebelow G. Oral immunisation of wild boar against classical swine fever: evaluation of the first field study in Germany. Vet Microbiol. 2000; 73:239–252.
Article12. Martinez-Lopez B, Carpenter TE, Sanchez-Vizcaino JM. Risk assessment and cost-effectiveness analysis of Aujeszky's disease virus introduction through breeding and fattening pig movements into Spain. Prev Vet Med. 2009; 90:10–16.
Article13. Miller GY, Tsai JS, Forster DL. Benefit-cost analysis of the national pseudorabies virus eradication program. J Am Vet Med Assoc. 1996; 208:208–213.14. World Organisation for Animal Health. Manual of diagnostic tests and vaccines for terrestrial animals (mammals, birds and bees). 7th ed. Paris: World Organisation for Animal Health;2012. p. 97–111.15. Maresch C, Lange E, Teifke JP, et al. Oral immunization of wild boar and domestic pigs with attenuated live vaccine protects against Pseudorabies virus infection. Vet Microbiol. 2012; 161:20–25.
Article16. An TQ, Peng JM, Tian ZJ, et al. Pseudorabies virus variant in Bartha-K61-vaccinated pigs, China, 2012. Emerg Infect Dis. 2013; 19:1749–1755.
Article17. Jakubik J. Comparative susceptibility of rabbits, rats, mice and pigs to infection with Aujeszky diseases virus (ADV) in the development of an efficacy test for ADV vaccines. Zentralbl Veterinarmed B. 1977; 24:764–766.
Article18. Alva-Valdes R, Glock RD, Kluge JP, Hill HT. The effects of challenge on the humoral and cellular immune responses in pseudorabies vaccinated swine. Can J Comp Med. 1983; 47:451–455.19. Pol JM, Gielkens AL, van Oirschot JT. Comparative pathogenesis of three strains of pseudorabies virus in pigs. Microb Pathog. 1989; 7:361–371.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immune responses in pigs and cattle vaccinated with half-volume foot-and-mouth disease vaccine
- Development of inactivated Akabane and bovine ephemeral fever vaccine for cattle
- Antibody Response in Cattle and Guinea Pigs Inoculated with Rabies Vaccines
- Inactivated genotype 1 Japanese encephalitis vaccine for swine
- Augmented immune responses in pigs immunized with an inactivated porcine reproductive and respiratory syndrome virus containing the deglycosylated glycoprotein 5 under field conditions