J Bacteriol Virol.
2014 Mar;44(1):67-74. 10.4167/jbv.2014.44.1.67.
Antibody Response in Cattle and Guinea Pigs Inoculated with Rabies Vaccines
- Affiliations
-
- 1Viral disease division, Animal and Plant Quarantine Agency, MAFRA, Anyang, Korea. yangdk@korea.kr
- 2Gangwon-do Veterinary Service Laboratory, Sokcho, Korea.
- 3College of Veterinary Medicine, Kangwon National University, Chun-Cheon, Korea.
- KMID: 2135359
- DOI: http://doi.org/10.4167/jbv.2014.44.1.67
Abstract
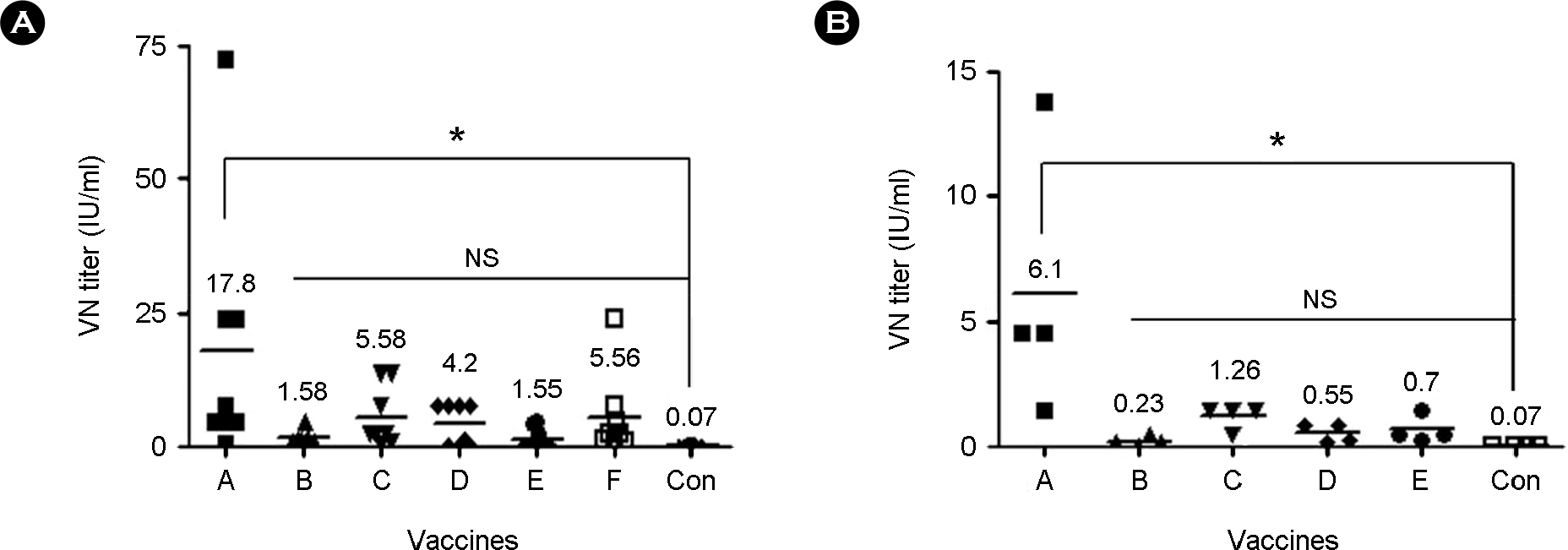
- One hundred ninety-five rabies cases in cattle were identified in South Korea since 1993. As most of rabies cases have a relation to rabid Korean raccoon dogs (Nyctereutes procyonoides koreensis), vaccination to animals including cattle is mandatory in rabies risk region. In order to minimize fatal rabies in animals, eradication policy of the disease has been achieved by controlling reservoirs and by mass vaccination. In this study, we compared the antibody response in cattle and guinea pigs inoculated with rabies vaccines commercially available in Korea. Each group of cattle in Gangwon-do was vaccinated intramuscularly with either one of five commercial inactivated vaccines or a live attenuated rabies vaccine (designated as A to F). Serum samples at the time of vaccination and four weeks post vaccination were obtained from the cattle and guinea pigs and were analyzed with virus neutralizing assay (VNA). Each group of cattle inoculating rabies vaccines showed significant virus neutralizing antibody titers (p < 0.05) ranging from 1.55 to 17.8 mean IU/ml compared with the non-vaccinated cattle and guinea pigs inoculated with 1/20 dose of vaccine showed relatively low VN antibody titers ranging from 0.23 to 6.1 mean IU/ml. All cattle immunized with A, C and F showed high VN antibody titers over 0.5 IU/ml and 62.5% and 37.5% of cattle inoculated with D and E showed protective antibody titer, respectively. This finding suggests that the inactivated or live attenuated rabies vaccination commercially available in Korea could induce protective antibody response in Korean cattle, but sero-conversion rate and sero-positive rate showing VN antibody titer over 0.5 IU/ml depend on vaccines.
Keyword
MeSH Terms
Figure
Reference
-
1). World Health Organization (WHO). WHO expert committee on rabies. World Health Organ Tech Rep Ser. 1992; 824:1–84.2). Johnson N, Black C, Smith J, Un H, McElhinney LM, Aylan O, et al. Rabies emergence among foxes in Turkey. J Wildl Dis. 2003; 39:262–70.
Article3). Smith JS, Orciari LA, Yager PA, Seidel HD, Warner CK. Epidemiologic and historical relationships among 87 rabies virus isolates as determined by limited sequence analysis. J Infect Dis. 1992; 166:296–307.
Article4). Sterner RT, Meltzer MI, Shwiff SA, Slate D. Tactics and economics of wildlife oral rabies vaccination, Canada and the United States. Emerg Infect Dis. 2009; 15:1176–84.
Article5). Streicker DG, Altizer SM, Velasco-Villa A, Rupprecht CE. Variable evolutionary routes to host establishment across repeated rabies virus host shifts among bats. Proc Natl Acad Sci U S A. 2012; 109:19715–20.
Article6). Yang DK, Kim SY, Oh YI, Lee JA, Cho SD, Lee KW, et al. Epidemiological Characteristics of Rabies in South Korea from January 2004 to March 2011. J Bacteriol Virol. 2011; 41:165–71.
Article7). Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, et al. Field virology. Philadelphia: Lippincott Williams & Wilkins;2001. p. 1221–77.8). Joo YS, Lee JH, Lee KK, Bang HA, Lee WC. Retrospective study of extensive vaccination programs for canine rabies control and public health in Korea. Jpn J Infect Dis. 2011; 64:513–5.9). Kim CH, Lee CG, Yoon HC, Nam HM, Park CK, Lee JC, et al. Rabies, an emerging disease in Korea. J Vet Med B Infect Dis Vet Public Health. 2006; 53:111–5.
Article10). Wunderli PS, Dreesen DW, Miller TJ, Baer GM. The rabies peripheral challenge test: more accurate determination of vaccine potency. Vaccine. 2006; 24:7115–23.
Article11). Krämer B, Bruckner L, Daas A, Milne C. Collaborative study for validation of a serological potency assay for rabies vaccine (inactivated) for veterinary use. Pharmeur Bio Sci Notes. 2010; 2010:37–55.12). Gamoh K, Shimazaki Y, Senda M, Makie H, Itoh O, Muramatsu M, et al. Establishment of a potency test by ELISA for a rabies vaccine for animal use in Japan. J Vet Med Sci. 2003; 65:685–8.
Article13). Kamphuis E, Krämer B, Schildger H, Duchow K. Potency testing of inactivated rabies vaccines using a serological method. Dev Biol (Basel). 2012; 134:23–7.14). Kolotvina PV, Gribencha SV, Kasatkin VV, Romanova LN, Movsesiants AA, Shkol'nik RIa, et al. The efficacy of a cultured rabies vaccine studied on guinea pigs previously infected with the rabies street virus. Zh Mikrobiol Epidemiol Immunobiol. 1990; 6:58–64.15). Yang DK, Oh YI, Cho SD, Kang HK, Lee KW, Kim YH, et al. Molecular Identification of the Vaccine Strain from the Inactivated Rabies Vaccine. J Bacteriol Virol. 2011; 41:47–54.
Article16). Minke JM, Bouvet J, Cliquet F, Wasniewski M, Guiot AL, Lemaitre L, et al. Comparison of antibody responses after vaccination with two inactivated rabies vaccines. Vet Microbiol. 2009; 133:283–6.
Article17). Aubert MF. Practical significance of rabies antibodies in cats and dogs. Rev Sci Tech. 1992; 11:735–60.
Article18). Brown CL, Rupprecht CE. Vaccination of free-ranging Pennsylvania raccoons (Procyon lotor) with inactivated rabies vaccine. J Wildl Dis. 1990; 26:253–7.19). Cliquet F, Aubert M, Sagné L. Development of a fluorescent antibody virus neutralisation test (FAVN test) for the quantitation of rabies-neutralising antibody. J Immunol Methods. 1998; 212:79–87.
Article20). Kuzmin IV, Botvinkin AD, McElhinney LM, Smith JS, Orciari LA, Hughes GJ, et al. Molecular epidemiology of terrestrial rabies in the former Soviet Union. J Wildl Dis. 2004; 40:617–31.
Article21). Cliquet F, Barrat J, Guiot AL, Caël N, Boutrand S, Maki J, et al. Efficacy and bait acceptance of vaccinia vectored rabies glycoprotein vaccine in captive foxes (Vulpes vulpes), raccoon dogs (Nyctereutes procyonoides) and dogs (Canis familiaris). Vaccine. 2008; 26:4627–38.22). Li J, McGettigan JP, Faber M, Schnell MJ, Dietzschold B. Infection of monocytes or immature dendritic cells (DCs) with an attenuated rabies virus results in DC maturation and a strong activation of the NFkappaB signaling pathway. Vaccine. 2008; 26:419–26.23). Monaco F, Franchi PM, Lelli R. Studies on an inactivated vaccine against rabies virus in domestic animals. Dev Biol (Basel). 2006; 125:233–9.24). Lalosević D, Lalosević V, Lazarević-Ivanc LJ, Knezević I. BHK-21 cell culture rabies vaccine: immunogenicity of a candidate vaccine for humans. Dev Biol (Basel). 2008; 131:421–9.25). Hanlon CA, Niezgoda M, Hamir AN, Schumacher C, Koprowski H, Rupprecht CE. First North American field release of a vaccinia-rabies glycoprotein recombinant virus. J Wildl Dis. 1998; 34:228–39.
Article26). Piza AT, Pieri KM, Lusa GM, Caporale GM, Terreran MT, Machado LA, et al. Effect of the contents and form of rabies glycoprotein on the potency of rabies vaccination in cattle. Mem Inst Oswaldo Cruz. 2002; 97:265–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunogenicity of an inactivated rabies vaccine for animals derived from the recombinant ERAGS strain
- Development of inactivated Akabane and bovine ephemeral fever vaccine for cattle
- A genetically modified rabies vaccine (ERAGS) induces protective immunity in dogs and cattle
- Antibody Response in Korean Raccoon Dogs Inoculated with Inactivated Rabies Vaccines
- The Comparison of Cellular Immune Reactions in Mycobacterium Tuberculosis-Inoculated Guinea Pigs and Rabbits