J Vet Sci.
2017 Aug;18(S1):323-331. 10.4142/jvs.2017.18.S1.323.
Immune responses in pigs and cattle vaccinated with half-volume foot-and-mouth disease vaccine
- Affiliations
-
- 1Animal and Plant Quarantine Agency, Gimcheon 39660, Korea. parkjhvet@korea.kr
- 2College of Veterinary Medicine (BK21 Plus Program), Chungnam National University, Daejon 34134, Korea.
- KMID: 2412557
- DOI: http://doi.org/10.4142/jvs.2017.18.S1.323
Abstract
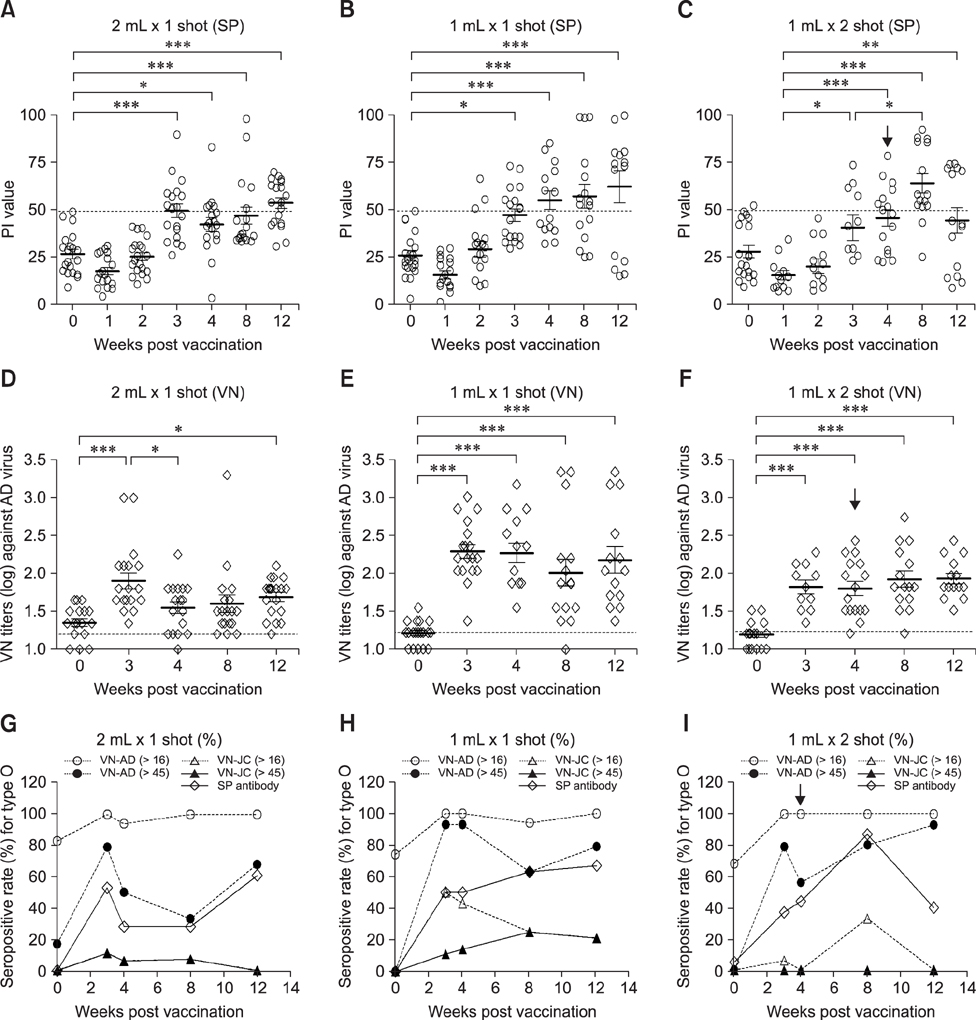
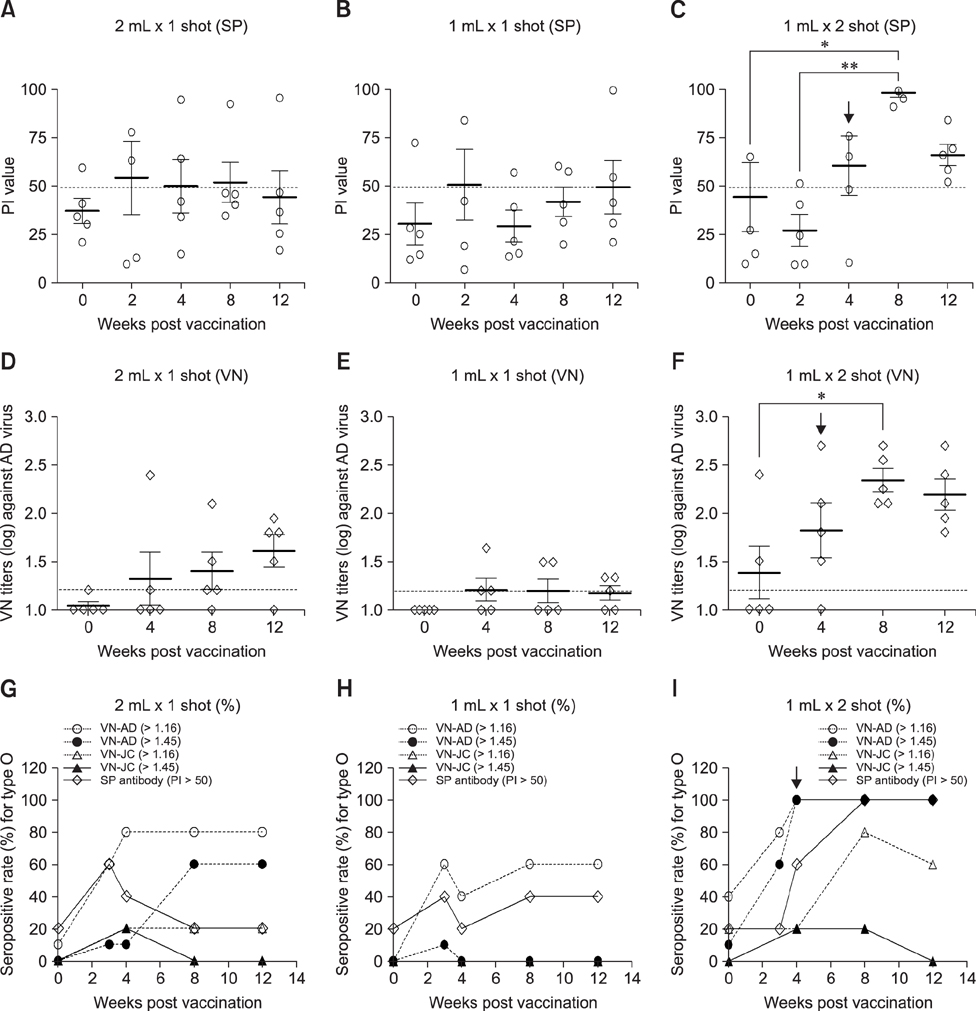
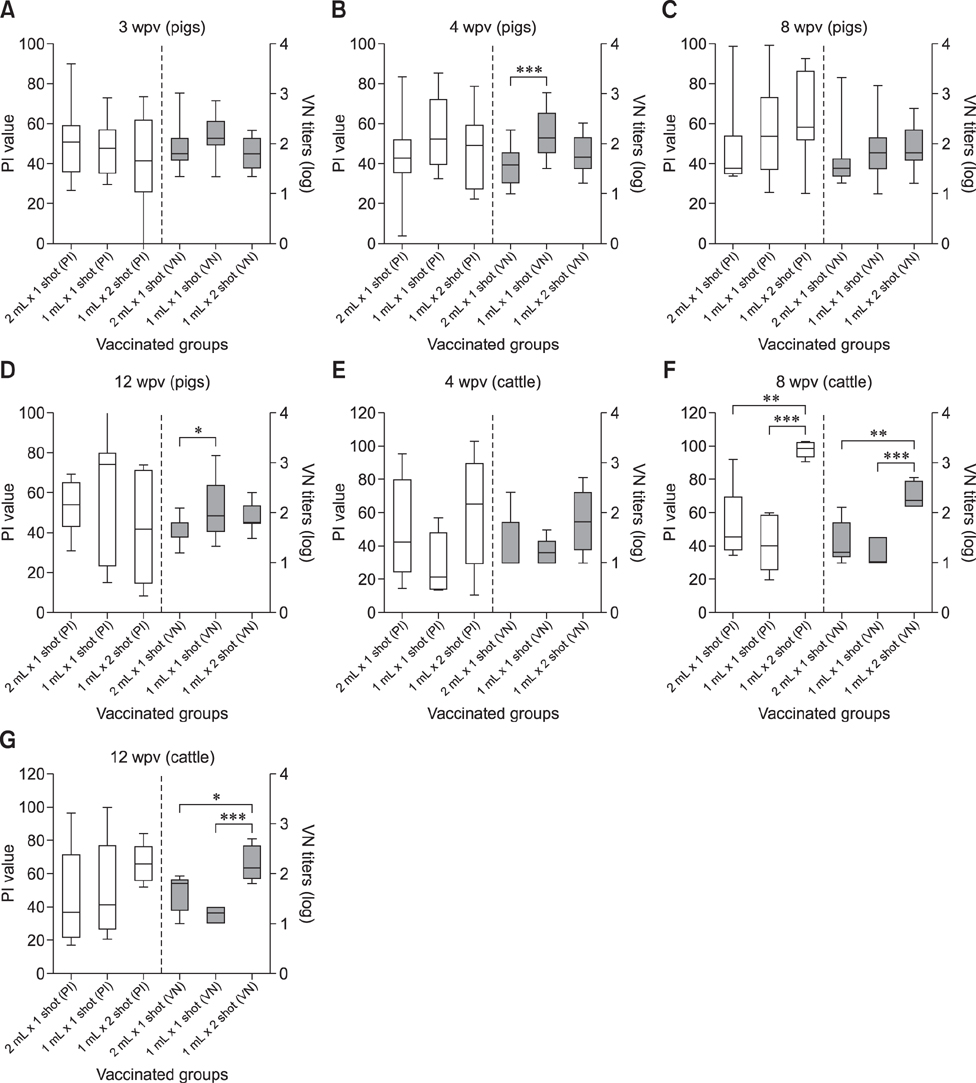
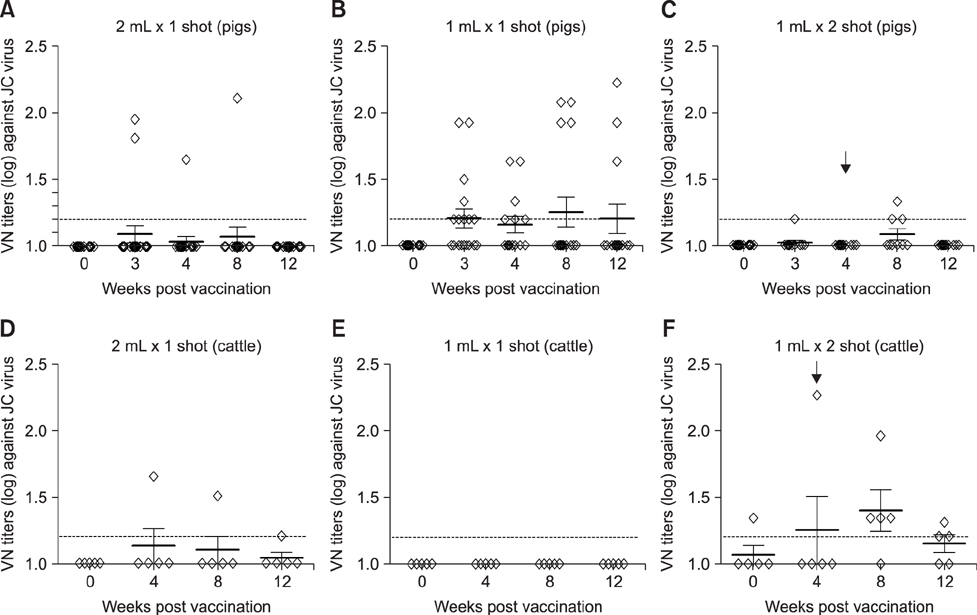
- With the current commercial foot-and-mouth disease vaccine, inoculating twice increases the formation of denatured meat due to granuloma or residual adjuvant at the injection site in pigs, resulting in economic loss. Therefore, we investigated protective antibody levels after reducing the amount of adjuvant in the vaccine. Field applicability of the experimental vaccine, made with a new adjuvant ISA 201, was tested by vaccinating farm animals with half-volume doses (1 mL/animal) of commercial vaccine and monitoring their immunogenicity. Among pigs, the group that received a half-volume dose showed similar or higher titers of structural protein antibody and neutralizing antibody than those receiving the standard dose (2 mL). In pigs, the durable effects of antibody titer of the reduced vaccine volume did not diminish up to the time of slaughter. Among cattle, boosting with a second 1 mL vaccine increased virus neutralizing antibody for the protective effects. The boosting effects were more marked in cattle than in pigs. The immune responses differed between species with the effect of the half-volume vaccination being lower in cattle than in pigs. In conclusion, the immune response to the half-volume vaccine was similar to that from the standard volume vaccine in pigs, but not in cattle.
Keyword
MeSH Terms
-
Animals
Antibody Formation/immunology
Cattle
Cattle Diseases/immunology/*prevention & control/virology
Dose-Response Relationship, Immunologic
Foot-and-Mouth Disease/immunology/*prevention & control
Foot-and-Mouth Disease Virus/*immunology
Swine
Swine Diseases/immunology/*prevention & control/virology
Viral Vaccines/administration & dosage/immunology/*therapeutic use
Viral Vaccines
Figure
Cited by 2 articles
-
Needleless intradermal vaccination for foot-and-mouth disease induced granuloma-free effective protection in pigs
Ji-Hyeon Hwang, Kwang-Nyeong Lee, Su-Mi Kim, Gyeongmin Lee, Yoonjung Moon, Byounghan Kim, Jong-Soo Lee, Jong-Hyeon Park
J Vet Sci. 2019;20(3):. doi: 10.4142/jvs.2019.20.e29.New foot-and-mouth disease vaccine, O JC-R, induce complete protection to pigs against SEA topotype viruses occurred in South Korea, 2014–2015
Hye-Eun Jo, Mi-Kyeong Ko, Joo-Hyung Choi, Sung Ho Shin, Hyundong Jo, Su-Hwa You, Min Ja Lee, Su-Mi Kim, Byounghan Kim, Jong-Hyeon Park
J Vet Sci. 2019;20(4):. doi: 10.4142/jvs.2019.20.e42.
Reference
-
1. Barnett PV, Pullen L, Williams L, Doel TR. International bank for foot-and-mouth disease vaccine: assessment of Montanide ISA 25 and ISA 206, two commercially available oil adjuvants. Vaccine. 1996; 14:1187–1198.
Article2. Choi SH, Park SI. Economic burden of foot-and-mouth disease vaccination-induced injection site lesions in slaughtered pigs and its causal relationship. J Prev Vet Med. 2015; 39:153–156.
Article3. Couch RB, Winokur P, Edwards KM, Black S, Atmar RL, Stapleton JT, Kissner JM, Shinefield H, Denny TN, Bybel MJ, Newman FK, Yan L. National Institute of Allergy and Infectious Diseases Smallpox Vaccine Study Group. Reducing the dose of smallpox vaccine reduces vaccine-associated morbidity without reducing vaccination success rates or immune responses. J Infect Dis. 2007; 195:826–832.
Article4. Dekker A, Chénard G, Stockhofe N, Eblé PL. Proper timing of foot-and-mouth disease vaccination of piglets with maternally derived antibodies will maximize expected protection levels. Front Vet Sci. 2016; 3:52.
Article5. Fowler VL, Knowles NJ, Paton DJ, Barnett PV. Marker vaccine potential of a foot-and-mouth disease virus with a partial VP1 G-H loop deletion. Vaccine. 2010; 28:3428–3434.
Article6. Fukai K, Morioka K, Yamada M, Nishi T, Yoshida K, Kitano R, Yamazoe R, Kanno T. Comparative performance of fetal goat tongue cell line ZZ-R 127 and fetal porcine kidney cell line LFBK-αvβ6 for Foot-and-mouth disease virus isolation. J Vet Diagn Invest. 2015; 27:516–521.
Article7. Ibrahim Eel-S, Gamal WM, Hassan AI, Mahdy Sel-D, Hegazy AZ, Abdel-Atty MM. Comparative study on the immunopotentiator effect of ISA 201, ISA 61, ISA 50, ISA 206 used in trivalent foot and mouth disease vaccine. Vet World. 2015; 8:1189–1198.
Article8. Khorasani A, Madadgar O, Soleimanjahi H, Keyvanfar H, Mahravani H. Evaluation of the efficacy of a new oil-based adjuvant ISA 61 VG FMD vaccine as a potential vaccine for cattle. Iran J Vet Res. 2016; 17:8–12.9. Koh WM, Bogich T, Siegel K, Jin J, Chong EY, Tan CY, Chen MI, Horby P, Cook AR. The epidemiology of hand, foot and mouth disease in Asia: a systematic review and analysis. Pediatr Infect Dis J. 2016; 35:e285–e300.10. McKercher PD, Graves JH. A review of the current status of oil adjuvants in foot-and-mouth disease vaccines. Dev Biol Stand. 1976; 35:107–112.11. Park JH, Lee KN, Kim SM, Lee HS, Ko YJ, Tark DS, Shin YK, Seo MG, Kim B. Reemergence of foot-and-mouth disease, South Korea, 2000-2011. Emerg Infect Dis. 2014; 20:2158–2161.
Article12. Park JH, Tark D, Lee KN, Lee SY, Ko MK, Lee HS, Kim SM, Ko YJ, Seo MG, Chun JE, Lee MH, Kim B. Novel foot-and-mouth disease virus in Korea, July-August 2014. Clin Exp Vaccine Res. 2016; 5:83–87.
Article13. Park JN, Lee SY, Chu JQ, Lee YJ, Kim RH, Lee KN, Kim SM, Tark DS, Kim B, Park JH. Protection to homologous and heterologous challenge in pigs immunized with vaccine against foot-and-mouth disease type O caused an epidemic in East Asia during 2010/2011. Vaccine. 2014; 32:1882–1889.
Article14. Park ME, Lee SY, Kim RH, Ko MK, Lee KN, Kim SM, Kim BK, Lee JS, Kim B, Park JH. Enhanced immune responses of foot-and-mouth disease vaccine using new oil/gel adjuvant mixtures in pigs and goats. Vaccine. 2014; 32:5221–5227.
Article15. Park ME, Lee SY, Kim RH, Ko MK, Park JN, Lee KN, Kim SM, Choi JH, You SH, Kim B, Lee JS, Park JH. Altered adjuvant of foot-and-mouth disease vaccine improves immune response and protection from virus challenge. Trial Vaccinol. 2016; 5:97–104.
Article16. Patil PK, Sajjanar CM, Natarajan C, Bayry J. Neutralizing antibody responses to foot-and-mouth disease quadrivalent (type O, A, C and Asia 1) vaccines in growing calves with pre-existing maternal antibodies. Vet Microbiol. 2014; 169:233–235.
Article17. Xu M, Zuest R, Velumani S, Tukijan F, Toh YX, Appanna R, Tan EY, Cerny D, MacAry P, W CI, Fink K. A potent neutralizing antibody with therapeutic potential against all four serotypes of dengue virus. NPJ Vaccines. 2017; 2:2.
Article18. Yamanaka M, Hiramatsu K, Hirahara T, Okabe T, Nakai M, Sasaki N, Goto N. Pathological studies on local tissue reactions in guinea pigs and rats caused by four different adjuvants. J Vet Med Sci. 1992; 54:685–692.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Genetic identification and serological evaluation of commercial inactivated foot-and-mouth disease virus vaccine in pigs
- New foot-and-mouth disease vaccine, O JC-R, induce complete protection to pigs against SEA topotype viruses occurred in South Korea, 2014–2015
- Needleless intradermal vaccination for foot-and-mouth disease induced granuloma-free effective protection in pigs
- Blood parameter changes in Korean traditional calves and pigs after foot-and-mouth disease vaccination
- Effect of simultaneous administration of foot-and-mouth disease (FMD) and anthrax vaccines on antibody response to FMD in sheep