Yonsei Med J.
2017 May;58(3):581-591. 10.3349/ymj.2017.58.3.581.
Potential Antitumor Activity of SIM-89 in Non-Small Cell Lung Cancer Cells
- Affiliations
-
- 1Department of Pulmonary, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China. hanbh520@163.com
- 2Department of Basic Research, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China.
- 3Department of Biology, East China Normal University, Shanghai, China.
- KMID: 2419116
- DOI: http://doi.org/10.3349/ymj.2017.58.3.581
Abstract
- PURPOSE
c-Met and its ligand, hepatocyte growth factor (HGF), play a critical role in oncogenesis and metastatic progression. The aim of this study was to identify inhibited enzymogram and to test the antitumor activity of SIM-89 (a c-Met receptor tyrosine kinase inhibitor) in non-small cell lung cancer.
MATERIALS AND METHODS
Z"²-LYTE kinase assay was employed to screen the kinase enzymogram, and mechanism of action (MOA) analysis was used to identify the inhibited kinases. Cell proliferation was then analyzed by CCK8 assay, and cell migration was determined by transwell assay. The gene expression and the phosphorylation of c-Met were examined by realtime-PCR and western blotting, respectively. Finally, the secretion of HGF was detected by ELISA assay.
RESULTS
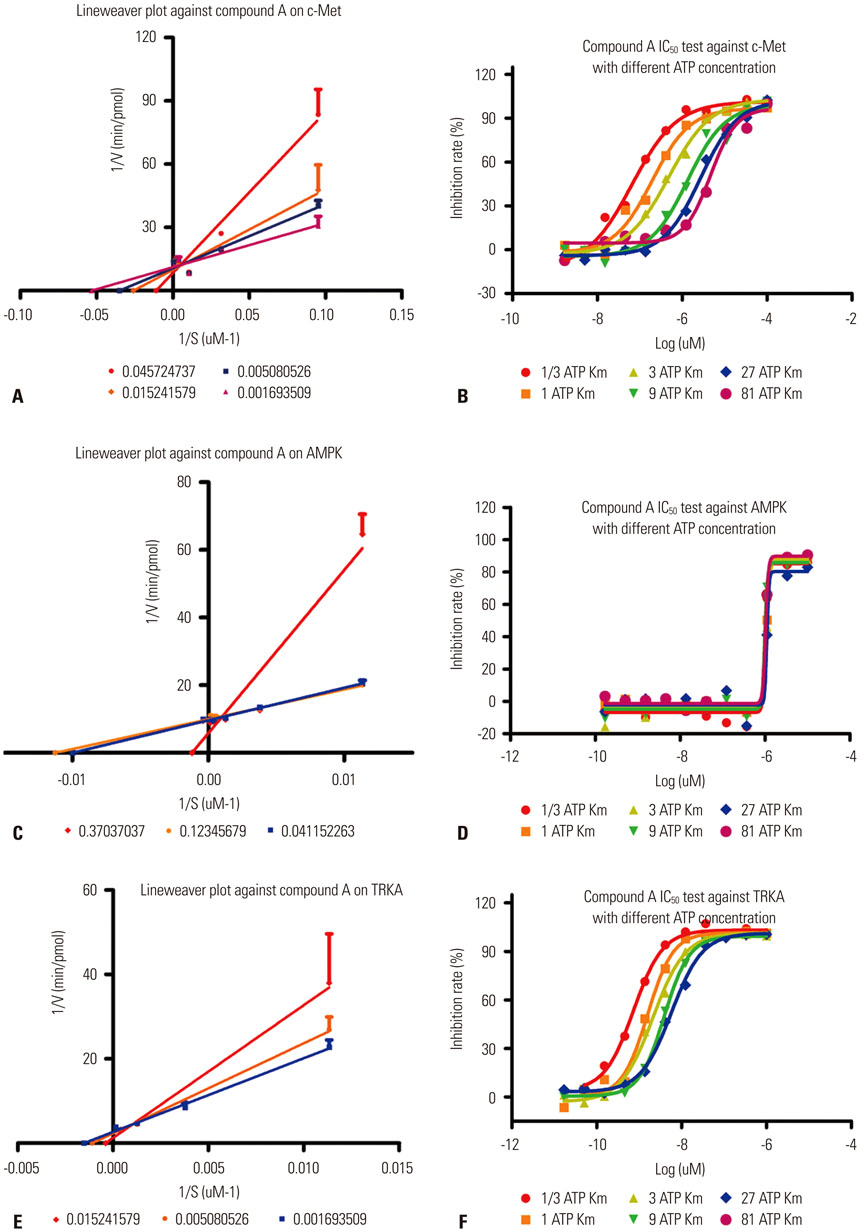
c-Met, activated protein kinase (AMPK), and tyrosine kinase A (TRKA) were inhibited by SIM-89 with the ICâ‚…â‚€ values of 297 nmol/L, 1.31 µmol/L, and 150.2 nmol/L, respectively. SIM-89 exerted adenosine triphosphate (ATP) competitive inhibition on c-Met. Moreover, the expressions of STAT1, JAK1, and c-Met in H460 cells were decreased by SIM-89 treatment, and c-Met phosphorylation was suppressed in A549, H441, H1299, and B16F10 cells by the treatment. In addition, SIM-89 treatment significantly decreased the level of HGF, which accounted for the activation of c-Met receptor tyrosine kinase. Finally, we showed cell proliferation inhibition and cell migration suppression in H460 and H1299 cells after SIM-89 treatment.
CONCLUSION
In conclusion, SIM-89 inhibits tumor cell proliferation, migration and HGF autocrine, suggesting it's potential antitumor activity.
Keyword
MeSH Terms
-
Antineoplastic Agents/*pharmacology
Blotting, Western
Carcinoma, Non-Small-Cell Lung/*drug therapy/enzymology/pathology
*Cell Line, Tumor
Cell Movement/drug effects
Cell Proliferation/*drug effects
Enzyme-Linked Immunosorbent Assay
Hepatocyte Growth Factor/metabolism
Humans
Lung Neoplasms/*drug therapy/enzymology/pathology
Phosphorylation
Protein Kinase Inhibitors/*pharmacology
Proto-Oncogene Proteins c-met/*antagonists & inhibitors/*genetics/metabolism
Signal Transduction/drug effects
Xenograft Model Antitumor Assays
Antineoplastic Agents
Protein Kinase Inhibitors
Hepatocyte Growth Factor
Proto-Oncogene Proteins c-met
Figure
Reference
-
1. Stoker M, Gherardi E, Perryman M, Gray J. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature. 1987; 327:239–242.
Article2. Gherardi E, Stoker M. Hepatocytes and scatter factor. Nature. 1990; 346:228.
Article3. Boccaccio C, Comoglio PM. Invasive growth: a MET-driven genetic programme for cancer and stem cells. Nat Rev Cancer. 2006; 6:637–645.
Article4. Comoglio PM, Giordano S, Trusolino L. Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat Rev Drug Discov. 2008; 7:504–516.
Article5. Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003; 4:915–925.
Article6. Xiao GH, Jeffers M, Bellacosa A, Mitsuuchi Y, Vande Woude GF, Testa JR. Anti-apoptotic signaling by hepatocyte growth factor/Met via the phosphatidylinositol 3-kinase/Akt and mitogen-activated protein kinase pathways. Proc Natl Acad Sci U S A. 2001; 98:247–252.
Article7. Wang X, DeFrances MC, Dai Y, Pediaditakis P, Johnson C, Bell A, et al. A mechanism of cell survival: sequestration of Fas by the HGF receptor Met. Mol Cell. 2002; 9:411–421.8. Xin X, Yang S, Ingle G, Zlot C, Rangell L, Kowalski J, et al. Hepatocyte growth factor enhances vascular endothelial growth factor-induced angiogenesis in vitro and in vivo. Am J Pathol. 2001; 158:1111–1120.
Article9. Seiwert TY, Jagadeeswaran R, Faoro L, Janamanchi V, Nallasura V, El Dinali M, et al. The MET receptor tyrosine kinase is a potential novel therapeutic target for head and neck squamous cell carcinoma. Cancer Res. 2009; 69:3021–3031.
Article10. Christensen JG, Burrows J, Salgia R. c-Met as a target for human cancer and characterization of inhibitors for therapeutic intervention. Cancer Lett. 2005; 225:1–26.
Article11. Liu X, Yao W, Newton RC, Scherle PA. Targeting the c-MET signaling pathway for cancer therapy. Expert Opin Investig Drugs. 2008; 17:997–1011.
Article12. Zeng ZS, Weiser MR, Kuntz E, Chen CT, Khan SA, Forslund A, et al. c-Met gene amplification is associated with advanced stage colorectal cancer and liver metastases. Cancer Lett. 2008; 265:258–269.
Article13. Stabile LP, Lyker JS, Land SR, Dacic S, Zamboni BA, Siegfried JM. Transgenic mice overexpressing hepatocyte growth factor in the airways show increased susceptibility to lung cancer. Carcinogenesis. 2006; 27:1547–1555.
Article14. Chen JT, Lin TS, Chow KC, Huang HH, Chiou SH, Chiang SF, et al. Cigarette smoking induces overexpression of hepatocyte growth factor in type II pneumocytes and lung cancer cells. Am J Respir Cell Mol Biol. 2006; 34:264–273.
Article15. Cipriani NA, Abidoye OO, Vokes E, Salgia R. MET as a target for treatment of chest tumors. Lung Cancer. 2009; 63:169–179.
Article16. Ma PC, Jagadeeswaran R, Jagadeesh S, Tretiakova MS, Nallasura V, Fox EA, et al. Functional expression and mutations of c-Met and its therapeutic inhibition with SU11274 and small interfering RNA in non-small cell lung cancer. Cancer Res. 2005; 65:1479–1488.
Article17. McDermott U, Sharma SV, Dowell L, Greninger P, Montagut C, Lamb J, et al. Identification of genotype-correlated sensitivity to selective kinase inhibitors by using high-throughput tumor cell line profiling. Proc Natl Acad Sci U S A. 2007; 104:19936–19941.
Article18. Mao WF, Shao MH, Gao PT, Ma J, Li HJ, Li GL, et al. The important roles of RET, VEGFR2 and the RAF/MEK/ERK pathway in cancer treatment with sorafenib. Acta Pharmacol Sin. 2012; 33:1311–1318.
Article19. Sattler M, Reddy MM, Hasina R, Gangadhar T, Salgia R. The role of the c-Met pathway in lung cancer and the potential for targeted therapy. Ther Adv Med Oncol. 2011; 3:171–184.
Article20. Sattler M, Salgia R. The MET axis as a therapeutic target. Update Cancer Ther. 2009; 3:109–118.
Article21. Krishnaswamy S, Kanteti R, Duke-Cohan JS, Loganathan S, Liu W, Ma PC, et al. Ethnic differences and functional analysis of MET mutations in lung cancer. Clin Cancer Res. 2009; 15:5714–5723.
Article22. Okuda K, Sasaki H, Yukiue H, Yano M, Fujii Y. Met gene copy number predicts the prognosis for completely resected non-small cell lung cancer. Cancer Sci. 2008; 99:2280–2285.
Article23. Cappuzzo F, Marchetti A, Skokan M, Rossi E, Gajapathy S, Felicioni L, et al. Increased MET gene copy number negatively affects survival of surgically resected non-small-cell lung cancer patients. J Clin Oncol. 2009; 27:1667–1674.
Article24. Go H, Jeon YK, Park HJ, Sung SW, Seo JW, Chung DH. High MET gene copy number leads to shorter survival in patients with non-small cell lung cancer. J Thorac Oncol. 2010; 5:305–313.
Article25. Ma PC, Tretiakova MS, MacKinnon AC, Ramnath N, Johnson C, Dietrich S, et al. Expression and mutational analysis of MET in human solid cancers. Genes Chromosomes Cancer. 2008; 47:1025–1037.26. Tretiakova M, Salama AK, Karrison T, Ferguson MK, Husain AN, Vokes EE, et al. MET and phosphorylated MET as potential biomarkers in lung cancer. J Environ Pathol Toxicol Oncol. 2011; 30:341–354.
Article27. Ferraro D, Corso S, Fasano E, Panieri E, Santangelo R, Borrello S, et al. Pro-metastatic signaling by c-Met through RAC-1 and reactive oxygen species (ROS). Oncogene. 2006; 25:3689–3698.
Article28. Navab R, Liu J, Seiden-Long I, Shih W, Li M, Bandarchi B, et al. Co-overexpression of Met and hepatocyte growth factor promotes systemic metastasis in NCI-H460 non-small cell lung carcinoma cells. Neoplasia. 2009; 11:1292–1300.
Article29. Ravichandran K, Tyagi A, Deep G, Agarwal C, Agarwal R. Interleukin-1beta-induced iNOS expression in human lung carcinoma A549 cells: involvement of STAT and MAPK pathways. Indian J Exp Biol. 2011; 49:840–847.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Molecularly Targeted Therapy for Lung Cancer : Recent Topics
- Urushiol V Suppresses Cell Proliferation and Enhances Antitumor Activity of 5-FU in Human Colon Cancer Cells by Downregulating FoxM1
- Telomerase Activity in Non-small Cell Lung Cancer
- Overcoming the Intrinsic Gefitinib-resistance via Downregulation of AXL in Non-small Cell Lung Cancer
- Druggable Targets of Squamous Cell Lung Cancer