Yonsei Med J.
2018 Aug;59(6):801-805. 10.3349/ymj.2018.59.6.801.
Pathologically Confirmed Cerebral Amyloid Angiopathy with No Radiological Sign in a Patient with Early Onset Alzheimer's Disease
- Affiliations
-
- 1Department of Neurology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. yeshins@gmail.com
- 2Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. yl.suh@samsung.com
- 3Department of Neurology, Kangwon National University Hospital, Kangwon National University College of Medicine, Chuncheon, Korea.
- KMID: 2415541
- DOI: http://doi.org/10.3349/ymj.2018.59.6.801
Abstract
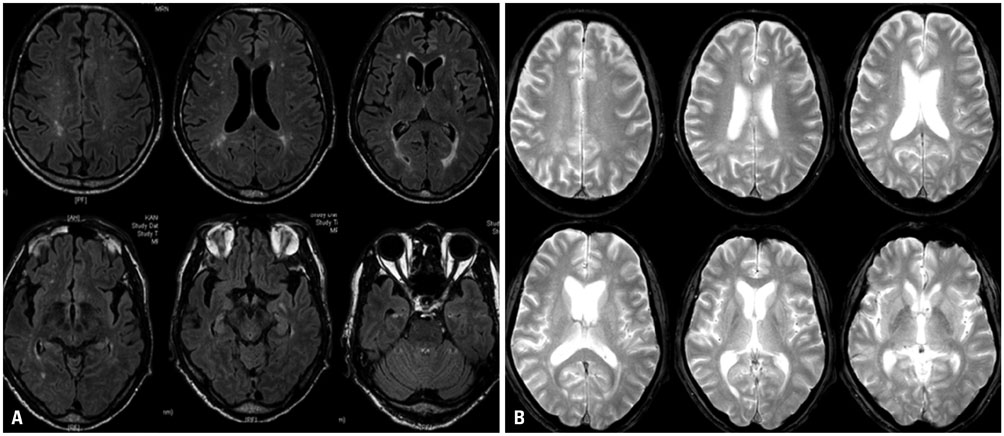
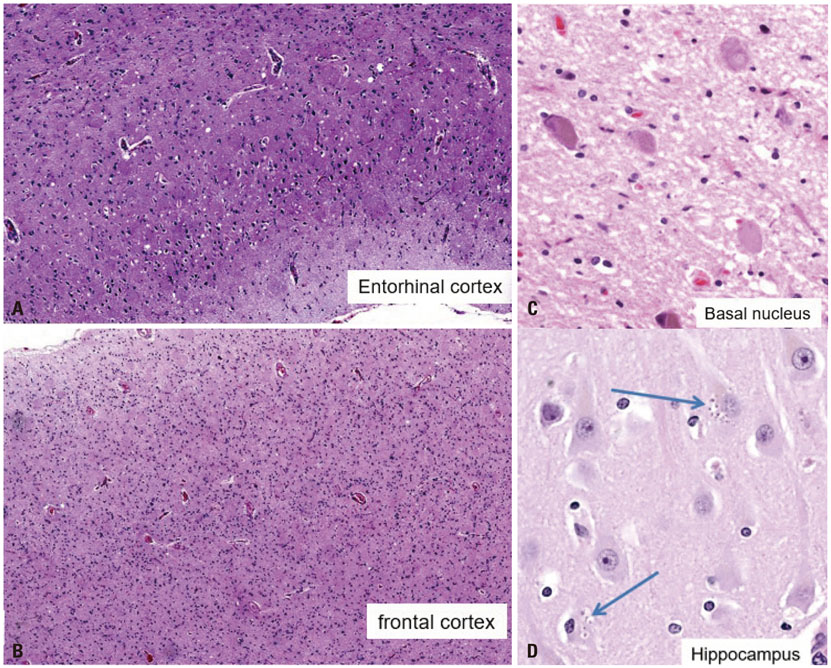
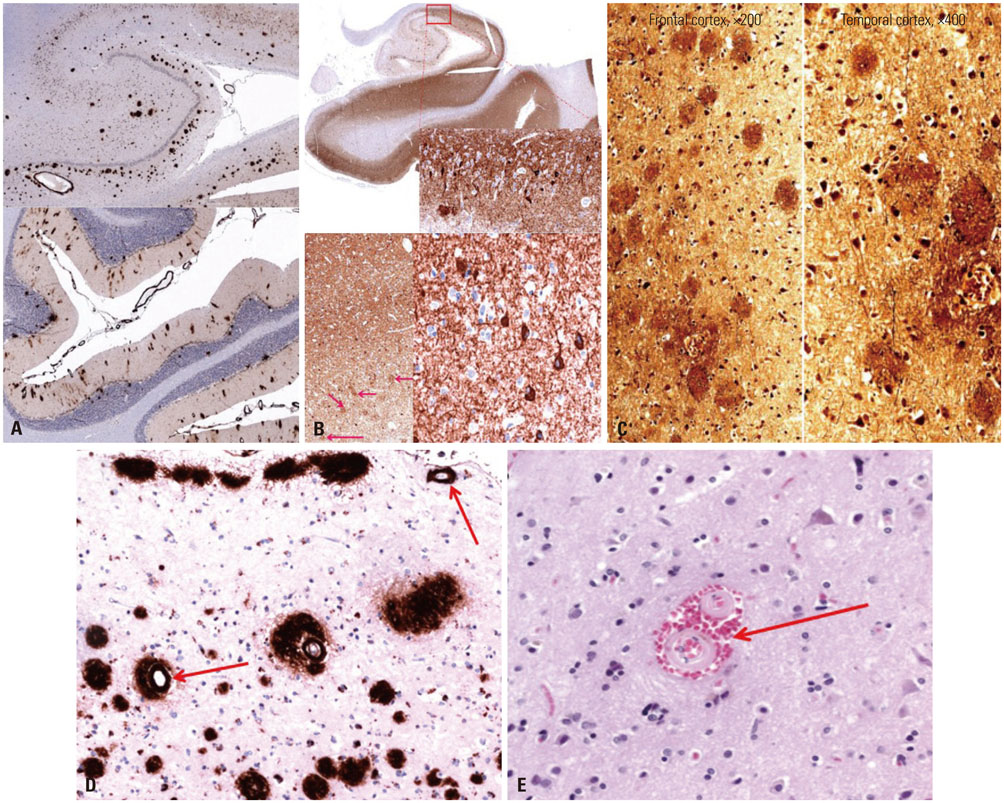
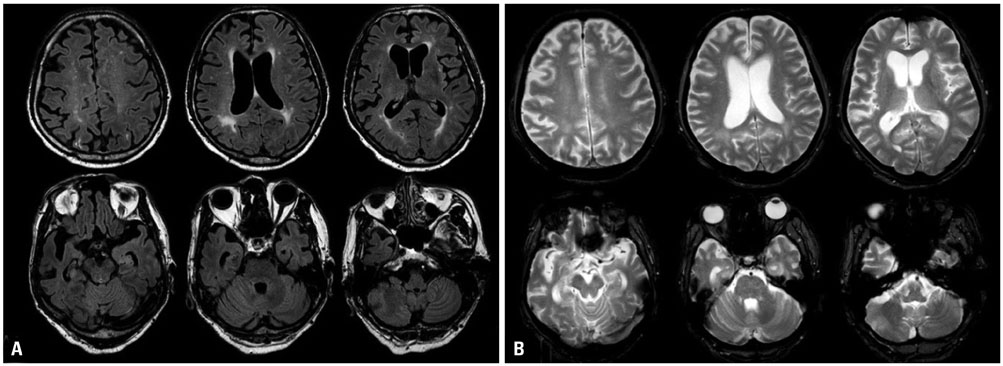
- Cerebral amyloid angiopathy (CAA) is associated with perivascular disruption, which is caused by progressive amyloid-beta (Aβ) deposition in vessels. Previous autopsy studies have shown that the prevalence of CAA in Alzheimer's disease (AD) is 70% to 90%. CAA is principally characterized by restricted lobar microbleeds (MBs), which can be detected by gradient-echo T2* (GRE) and susceptibility-weighted imaging (SWI). We herein report on a 62-year-old man who presented with 8 years of memory impairment. The apolipoprotein E (APOE) genotype was ε4/ε4, and a brain GRE performed 28 months before death revealed mild atrophy and no MBs. At autopsy, the patient scored "A3, B3, C3" according to the National Institute on Aging-Alzheimer's Association guidelines; the patient thus exhibited a high level of AD neuropathological changes. Furthermore, immunohistochemical staining for Aβ showed antibody accumulation and severe cerebral amyloid angiopathic changes in numerous vessels with amyloid deposits. Our case suggests that radiological CAA markers, such as cerebral microbleed (CMB) or cerebral superficial siderosis, may not suffice to detect amyloid angiopathy in cerebral vessels. CAA should therefore be considered as a combined pathology in APOE ε4 homozygotes with AD, even if such patients do not exhibit CMB on MRI.
MeSH Terms
Figure
Reference
-
1. Mendel TA, Wierzba-Bobrowicz T, Lewandowska E, Stępień T, Szpak GM. The development of cerebral amyloid angiopathy in cerebral vessels. A review with illustrations based upon own investigated post mortem cases. Pol J Pathol. 2013; 64:260–267.
Article2. Attems J, Jellinger KA, Lintner F. Alzheimer's disease pathology influences severity and topographical distribution of cerebral amyloid angiopathy. Acta Neuropathol. 2005; 110:222–231.
Article3. Ellis RJ, Olichney JM, Thal LJ, Mirra SS, Morris JC, Beekly D, et al. Cerebral amyloid angiopathy in the brains of patients with Alzheimer's disease: the CERAD experience, Part XV. Neurology. 1996; 46:1592–1596.
Article4. Piazza F, Greenberg SM, Savoiardo M, Gardinetti M, Chiapparini L, Raicher I, et al. Anti-amyloid β autoantibodies in cerebral amyloid angiopathy-related inflammation: implications for amyloid-modifying therapies. Ann Neurol. 2013; 73:449–458.
Article5. DiFrancesco JC, Longoni M, Piazza F. Anti-Aβ autoantibodies in amyloid related imaging abnormalities (ARIA): candidate biomarker for immunotherapy in Alzheimer's disease and cerebral amyloid angiopathy. Front Neurol. 2015; 6:207.
Article6. Pantoni L, Basile AM, Pracucci G, Asplund K, Bogousslavsky J, Chabriat H, et al. Impact of age-related cerebral white matter changes on the transition to disability -- the LADIS study: rationale, design and methodology. Neuroepidemiology. 2005; 24:51–62.
Article7. McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011; 7:263–269.
Article8. Olichney JM, Hansen LA, Hofstetter CR, Grundman M, Katzman R, Thal LJ. Cerebral infarction in Alzheimer's disease is associated with severe amyloid angiopathy and hypertension. Arch Neurol. 1995; 52:702–708.
Article9. Vonsattel JP, Myers RH, Hedley-Whyte ET, Ropper AH, Bird ED, Richardson EP Jr. Cerebral amyloid angiopathy without and with cerebral hemorrhages: a comparative histological study. Ann Neurol. 1991; 30:637–649.
Article10. Ringman JM, Sachs MC, Zhou Y, Monsell SE, Saver JL, Vinters HV. Clinical predictors of severe cerebral amyloid angiopathy and influence of APOE genotype in persons with pathologically verified Alzheimer disease. JAMA Neurol. 2014; 71:878–883.
Article11. Nandigam RN, Viswanathan A, Delgado P, Skehan ME, Smith EE, Rosand J, et al. MR imaging detection of cerebral microbleeds: effect of susceptibility-weighted imaging, section thickness, and field strength. AJNR Am J Neuroradiol. 2009; 30:338–343.
Article12. Haller S, Montandon ML, Lazeyras F, Scheffler M, Meckel S, Herrmann FR, et al. Radiologic-histopathologic correlation of cerebral microbleeds using pre-mortem and post-mortem MRI. PLoS One. 2016; 11:e0167743.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- In vivo Image of Cerebral Amyloid Angiopathy in an Alzheimer's Disease Mouse Model
- A Case of Cerebral Amyloid Angiopathy-related Intracerebral Hemorrhage
- Cerebral Amyloid Angiopathy: An Undeniable Small Vessel Disease
- Cerebral Amyloid Angiopathy: A report of two cases
- Multiple Recurrent Cerebral Hemorrhages Related to Cerebral Amyloid Angiopathy with Arterial Hypertension