J Korean Ophthalmol Soc.
2018 Jun;59(6):549-555. 10.3341/jkos.2018.59.6.549.
The Anti-fibrotic Effect of Nilotinib on Tenon's Capsule Fibroblasts in Vitro
- Affiliations
-
- 1Department of Ophthalmology, Chung-Ang University Hospital, Chung-Ang University College of Medicine, Seoul, Korea. njmoon@cau.ac.kr
- 2Department of Ophthalmology, Konyang University College of Medicine, Daejeon, Korea.
- KMID: 2413818
- DOI: http://doi.org/10.3341/jkos.2018.59.6.549
Abstract
- PURPOSE
To evaluate the anti-fibrotic effects of nilotinib on the survival of cultured human Tenon's capsule fibroblasts (HTFs).
METHODS
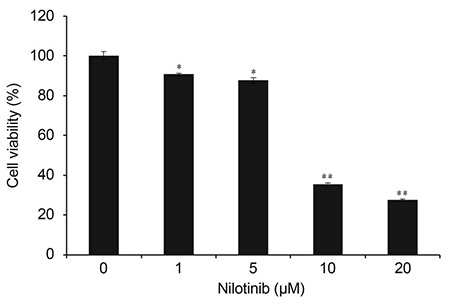
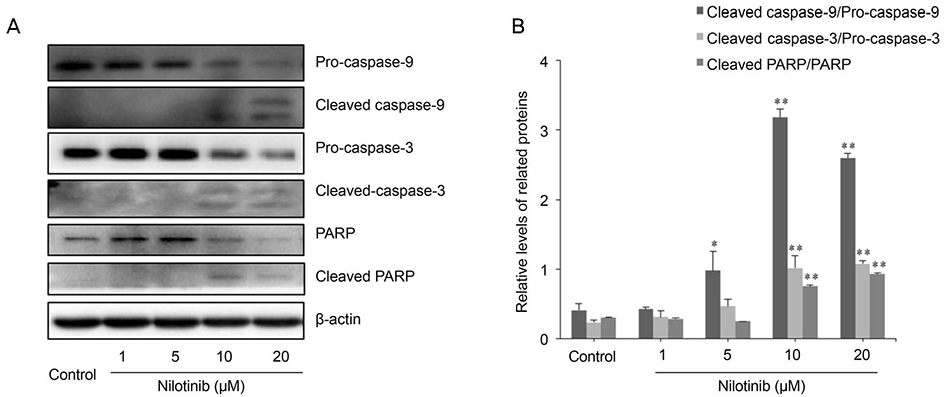
HTF primary cultures were obtained from samples following glaucoma surgery. Primarily cultured HTFs were exposed to 1, 5, 10, and 20 µM nilotinib for 24 hours. The effects of nilotinib on HTF proliferation and cell viability were determined using the 3-(4,5-dimethylthiazone-2-yl)-2,5-diphenyl tetrazolium (MTT) assay, and apoptosis was determined by flow cytometry using annexin-V/propidium iodide (PI) double staining. Apoptosis-related proteins were detected by western blotting.
RESULTS
The MTT assay showed that nilotinib induced an inhibition of HTF proliferation at concentrations of 10 and 20 µM (p < 0.001 and p < 0.001, respectively). Annexin V/PI double staining showed significantly increased apoptosis in cells treated with nilotinib. Nilotinib activated caspase-3, -9, and poly adenosine diphosphate ribose polymerase cleavage, and downregulated the expression of B-cell lymphoma-extra large and Bax, which indicated that nilotinib-induced apoptosis was partly mediated through the mitochondrial pathway. In addition, treatment with nilotinib decreased the expression of α-smooth muscle actin and transforming growth factor-β.
CONCLUSIONS
Nilotinib decreased cell survival of cultured HTFs and induced mitochondria-mediated apoptosis. The results suggested that nilotinib may exert antiproliferative effects on HTFs, making it a possible agent to control postoperative fibrosis in patients undergoing glaucoma surgery.
MeSH Terms
Figure
Reference
-
1. Eren K, Turgut B, Akin MM, Demir T. The suppression of wound healing response with sirolimus and sunitinib following experimental trabeculectomy in a rabbit model. Curr Eye Res. 2016; 41:367–376.
Article2. Xue H, McCauley RL, Zhang W, Martini DK. Altered interleukin-6 expression in fibroblasts from hypertrophic burn scars. J Burn Care Rehabil. 2000; 21:142–146.
Article3. Nakamura-Shibasaki M, Ko J, Takenaka J, et al. Matrix metalloproteinase and cytokine expression in Tenon fibroblasts during scar formation after glaucoma filtration or implant surgery in rats. Cell Biochem Funct. 2013; 31:482–488.
Article4. de Carvalho LE, Alves MR, da Silva MA, Gaal Vadas MF. Experimental strabismus surgery using triamcinolone: outcomes and effects on inflammatory response. Arq Bras Oftalmol. 2007; 70:209–215.5. Demirel S, Atilla H, Okcu Heper A, Erkam N. Effects of amniotic membrane on wound healing and adhesions in experimental strabismus surgery. Eur J Ophthalmol. 2009; 19:899–904.6. Frangouli O, Adams GG. The use of amniotic membrane for the management of fibrosis in complex strabismus surgery. Strabismus. 2013; 21:13–22.
Article7. Jung KI, Choi JS, Kim HK, Shin SY. Effects of an anti-transforming growth factor-beta agent (pirfenidone) on strabismus surgery in rabbits. Curr Eye Res. 2012; 37:770–776.8. Minguini N, Monteiro de Carvalho KM, Akaishi PM, De Luca IM. Histologic effect of mitomycin C on strabismus surgery in the rabbit. Invest Ophthalmol Vis Sci. 2000; 41:3399–3401.9. Mora JS, Sprunger DT, Helveston EM, Evan AP. Intraoperative sponge 5-fluorouracil to reduce postoperative scarring in strabismus surgery. J AAPOS. 1997; 1:92–97.10. Ozkan SB, Kir E, Culhaci N, Dayanir V. The effect of Seprafilm on adhesions in strabismus surgery-an experimental study. J AAPOS. 2004; 8:46–49.11. Kantarjian HM, Giles F, Gattermann N, et al. Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is effective in patients with Philadelphia chromosome-positive chronic myelogenous leukemia in chronic phase following imatinib resistance and intolerance. Blood. 2007; 110:3540–3546.
Article12. Shiha GE, Abu-Elsaad NM, Zalata KR, Ibrahim TM. Tracking anti-fibrotic pathways of nilotinib and imatinib in experimentally induced liver fibrosis: an insight. Clin Exp Pharmacol Physiol. 2014; 41:788–797.
Article13. Gordon JK, Martyanov V, Magro C, et al. Nilotinib (Tasigna(TM)) in the treatment of early diffuse systemic sclerosis: an open-label, pilot clinical trial. Arthritis Res Ther. 2015; 17:213.
Article14. Lemos DR, Babaeijandaghi F, Low M, et al. Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat Med. 2015; 21:786–794.
Article15. Rhee CK, Lee SH, Yoon HK, et al. Effect of nilotinib on bleomycin-induced acute lung injury and pulmonary fibrosis in mice. Respiration. 2011; 82:273–287.
Article16. Liu Y, Wang Z, Kwong SQ, et al. Inhibition of PDGF, TGF-beta, and Abl signaling and reduction of liver fibrosis by the small molecule Bcr-Abl tyrosine kinase antagonist Nilotinib. J Hepatol. 2011; 55:612–625.17. Assoian RK, Fleurdelys BE, Stevenson HC, et al. Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc Natl Acad Sci U S A. 1987; 84:6020–6024.
Article18. Assoian RK, Komoriya A, Meyers CA, et al. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem. 1983; 258:7155–7160.
Article19. Imanishi J, Kamiyama K, Iguchi I, et al. Growth factors: importance in wound healing and maintenance of transparency of the cornea. Prog Retin Eye Res. 2000; 19:113–129.
Article20. Sullivan KM, Lorenz HP, Meuli M, et al. A model of scarless human fetal wound repair is deficient in transforming growth factor beta. J Pediatr Surg. 1995; 30:198–202. discussion 202-3.
Article21. Shah M, Foreman DM, Ferguson MW. Control of scarring in adult wounds by neutralising antibody to transforming growth factor beta. Lancet. 1992; 339:213–214.22. Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003; 83:835–870.
Article23. Conte E, Gili E, Fagone E, et al. Effect of pirfenidone on proliferation, TGF-beta-induced myofibroblast differentiation and fibrogenic activity of primary human lung fibroblasts. Eur J Pharm Sci. 2014; 58:13–19.24. Hinz B, Celetta G, Tomasek JJ, et al. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell. 2001; 12:2730–2741.
Article25. Nagata S. Apoptosis by death factor. Cell. 1997; 88:355–365.
Article26. Susin SA, Lorenzo HK, Zamzami N, et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999; 397:441–446.
Article27. Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002; 2:647–656.
Article28. Willis SN, Fletcher JI, Kaufmann T, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007; 315:856–859.29. Wang CY, Mayo MW, Korneluk RG, et al. NF-kappaB anti-apoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998; 281:1680–1683.30. Bergmann MW, Loser P, Dietz R, von Harsdorf R. Effect of NF-kappa B Inhibition on TNF-alpha-induced apoptosis and downstream pathways in cardiomyocytes. J Mol Cell Cardiol. 2001; 33:1223–1232.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Gelrite on the Proliferation of Cultured Human Tenon's Capsule Fibroblasts
- Effect of Trusopt and Xalatan on the Proliferation of Cultured Subconjunctival Fibro blasts
- Comparison of the Effects Between Bevacizumab and Mitomycin C on the Survival of Fibroblasts
- Autophagy of Human Tenon's Capsule Fibroblasts Induced by Mitomycin-C
- Effect of Triamcinolone Acetonide on the Survival and Nitric Oxide Production in Cultured Human Tenon's Capsule Fibroblasts