J Vet Sci.
2016 Sep;17(3):279-287. 10.4142/jvs.2016.17.3.279.
Aristolochia manshuriensis Kom ethyl acetate extract protects against high-fat diet-induced non-alcoholic steatohepatitis by regulating kinase phosphorylation in mouse
- Affiliations
-
- 1Institute for Glycoscience, College of Natural Sciences, Wonkwang University, Iksan 54538, Korea. ykchoo@wonkwang.ac.kr
- 2Department of Biological Science, College of Natural Sciences, Wonkwang University, Iksan 54538, Korea.
- 3National Primate Research Center, Korea Institute of Bioscience and Biotechnology (KRIBB), Ochang 28116, Korea.
- KMID: 2413126
- DOI: http://doi.org/10.4142/jvs.2016.17.3.279
Abstract
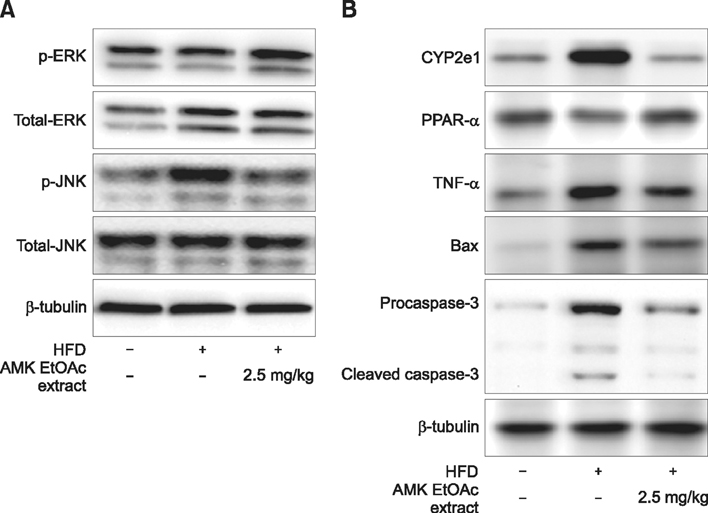
- Aristolochia manshuriensis Kom (AMK) is an herb used as a traditional medicine; however, it causes side effects such as nephrotoxicity and carcinogenicity. Nevertheless, AMK can be applied in specific ways medicinally, including via ingestion of low doses for short periods of time. Non-alcoholic steatohepatitis (NASH) induced the hepatocyte injury and inflammation. The protective effects of AMK against NASH are unclear; therefore, in this study, the protective effects of AMK ethyl acetate extract were investigated in a high-fat diet (HFD)-induced NASH model. We found decreased hepatic steatosis and inflammation, as well as increased levels of lipoproteins during AMK extract treatment. We also observed decreased hepatic lipid peroxidation and triglycerides, as well as suppressed hepatic expression of lipogenic genes in extract-treated livers. Treatment with extract decreased the activation of c-jun N-terminal kinase 1/2 (JNK1/2) and increased the phosphorylation of extracellular signal-regulated kinase 1/2 (ERK1/2). These results demonstrate that the protective effect of the extract against HFD-induced NASH occurred via reductions in reactive oxygen species production, inflammation suppression, and apoptosis related to the suppression of JNK1/2 activation and increased ERK1/2 phosphorylation. Taken together, these results indicate that that ethyl acetate extract of AMK has potential therapeutic effects in the HFD-induced NASH mouse model.
Keyword
MeSH Terms
-
Acetates/chemistry
Animals
Aristolochia/*chemistry
*Diet, High-Fat
Extracellular Signal-Regulated MAP Kinases/*genetics/metabolism
JNK Mitogen-Activated Protein Kinases/*genetics/metabolism
Liver/*drug effects/metabolism
Male
Mice
Mice, Inbred C57BL
Non-alcoholic Fatty Liver Disease/*drug therapy
Phosphorylation/drug effects
Plant Extracts/*pharmacology/*therapeutic use
Acetates
Plant Extracts
Extracellular Signal-Regulated MAP Kinases
JNK Mitogen-Activated Protein Kinases
Figure
Reference
-
1. Abdel-Razzak Z, Garlatti M, Aggerbeck M, Barouki R. Determination of interleukin-4-responsive region in the human cytochrome P450 2E1 gene promoter. Biochem Pharmacol. 2004; 68:1371–1381.
Article2. Angulo P, Lindor KD. Non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2002; 17:Suppl. S186–S190.
Article3. Bruce KD, Cagampang FR, Argenton M, Zhang J, Ethirajan PL, Burdge GC, Bateman AC, Clough GF, Poston L, Hanson MA, McConnell JM, Byrne CD. Maternal high-fat feeding primes steatohepatitis in adult mice offspring, involving mitochondrial dysfunction and altered lipogenesis gene expression. Hepatology. 2009; 50:1796–1808.
Article4. Chitturi S, Farrell GC. Etiopathogenesis of nonalcoholic steatohepatitis. Semin Liver Dis. 2001; 21:27–41.
Article5. Conde de la Rosa L, Schoemaker MH, Vrenken TE, Buist-Homan M, Havinga R, Jansen PL, Moshage H. Superoxide anions and hydrogen peroxide induce hepatocyte death by different mechanisms: involvement of JNK and ERK MAP kinases. J Hepatol. 2006; 44:918–929.
Article6. Cortez-Pinto H, Camilo ME. Non-alcoholic fatty liver disease/non-alcoholic steatohepatitis (NAFLD/NASH): diagnosis and clinical course. Best Pract Res Clin Gastroenterol. 2004; 18:1089–1104.
Article7. Czaja MJ, Liu H, Wang Y. Oxidant-induced hepatocyte injury from menadione is regulated by ERK and AP-1 signaling. Hepatology. 2003; 37:1405–1413.
Article8. Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998; 114:842–845.
Article9. Hirosumi J, Tuncman G, Chang L, Görgün CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002; 420:333–336.
Article10. Hotamisligil GS. Role of endoplasmic reticulum stress and c-Jun NH2-terminal kinase pathways in inflammation and origin of obesity and diabetes. Diabetes. 2005; 54:Suppl 2. S73–S78.11. Jin X, Zimmers TA, Perez EA, Pierce RH, Zhang Z, Koniaris LG. Paradoxical effects of short- and long-term interleukin-6 exposure on liver injury and repair. Hepatology. 2006; 43:474–484.
Article12. Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974; 11:151–169.
Article13. Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984; 21:130–132.14. Kelicen P, Tindberg N. Lipopolysaccharide induces CYP2E1 in astrocytes through MAP kinase kinase-3 and C/EBPβ and -δ. J Biol Chem. 2004; 279:15734–15742.
Article15. Klover PJ, Zimmers TA, Koniaris LG, Mooney RA. Chronic exposure to interleukin-6 causes hepatic insulin resistance in mice. Diabetes. 2003; 52:2784–2789.
Article16. Kugelmas M, Hill DB, Vivian B, Marsano L, McClain CJ. Cytokines and NASH: a pilot study of the effects of lifestyle modification and vitamin E. Hepatology. 2003; 38:413–419.
Article17. Kwak DH, Lee JH, Kim T, Ahn HS, Cho WK, Ha H, Hwang YH, Ma JY. Aristolochia manshuriensis Kom inhibits adipocyte differentiation by regulation of ERK1/2 and Akt pathway. PLoS One. 2012; 7:e49530.18. Martindale JL, Holbrook NJ. Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol. 2002; 192:1–15.
Article19. Pagano G, Pacini G, Musso G, Gambino R, Mecca F, Depetris N, Cassader M, David E, Cavallo-Perin P, Rizzetto M. Nonalcoholic steatohepatitis, insulin resistance, and metabolic syndrome: further evidence for an etiologic association. Hepatology. 2002; 35:367–372.
Article20. Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967; 70:158–169.21. Pathil A, Mueller J, Warth A, Chamulitrat W, Stremmel W. Ursodeoxycholyl lysophosphatidylethanolamide improves steatosis and inflammation in murine models of nonalcoholic fatty liver disease. Hepatology. 2012; 55:1369–1378.
Article22. Pessayre D, Fromenty B, Mansouri A. Mitochondrial injury in steatohepatitis. Eur J Gastroenterol Hepatol. 2004; 16:1095–1105.
Article23. Pozdzik AA, Salmon IJ, Husson CP, Decaestecker C, Rogier E, Bourgeade MF, Deschodt-Lanckman MM, Vanherweghem JL, Nortier JL. Patterns of interstitial inflammation during the evolution of renal injury in experimental aristolochic acid nephropathy. Nephrol Dial Transplant. 2008; 23:2480–2491.
Article24. Rahman SM, Schroeder-Gloeckler JM, Janssen RC, Jiang H, Qadri I, Maclean KN, Friedman JE. CCAAT/enhancing binding protein β deletion in mice attenuates inflammation, endoplasmic reticulum stress, and lipid accumulation in diet-induced nonalcoholic steatohepatitis. Hepatology. 2007; 45:1108–1117.
Article25. Sánchez-Alcázar JA, Schneider E, Martínez MA, Carmona P, Hernández-Muñoz I, Siles E, De La Torre P, Ruiz-Cabello J, García I, Solis-Herruzo JA. Tumor necrosis factor-α increases the steady-state reduction of cytochrome b of the mitochondrial respiratory chain in metabolically inhibited L929 cells. J Biol Chem. 2000; 275:13353–13361.
Article26. Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Clore JN. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001; 120:1183–1192.
Article27. Schattenberg JM, Singh R, Wang Y, Lefkowitch JH, Rigoli RM, Scherer PE, Czaja MJ. JNK1 but not JNK2 promotes the development of steatohepatitis in mice. Hepatology. 2006; 43:163–172.
Article28. Schwabe RF, Uchinami H, Qian T, Bennett BL, Lemasters JJ, Brenner DA. Differential requirement for c-Jun NH2-terminal kinase in TNF-α- and Fas-mediated apoptosis in hepatocytes. FASEB J. 2004; 18:720–722.
Article29. Shi LS, Kuo PC, Tsai YL, Damu AG, Wu TS. The alkaloids and other constituents from the root and stem of Aristolochia elegans. Bioorg Med Chem. 2004; 12:439–446.
Article30. Shirwaikar A, Somashekar AP, Udupa AL, Udupa SL, Somashekar S. Wound healing studies of Aristolochia bracteolata Lam. with supportive action of antioxidant enzymes. Phytomedicine. 2003; 10:558–562.
Article31. Singh R, Czaja MJ. Regulation of hepatocyte apoptosis by oxidative stress. J Gastroenterol Hepatol. 2007; 22:Suppl 1. S45–S48.
Article32. Ura S, Masuyama N, Graves JD, Gotoh Y. MST1-JNK promotes apoptosis via caspase-dependent and independent pathways. Genes Cells. 2001; 6:519–530.
Article33. Wieckowska A, Zein NN, Yerian LM, Lopez AR, McCullough AJ, Feldstein AE. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology. 2006; 44:27–33.
Article34. Yang L, Li X, Wang H. Possible mechanisms explaining the tendency towards interstitial fibrosis in aristolochic acid-induced acute tubular necrosis. Nephrol Dial Transplant. 2007; 22:445–456.
Article35. Yu J, Ip E, Dela Peña A, Hou JY, Sesha J, Pera N, Hall P, Kirsch R, Leclercq I, Farrell GC. COX-2 induction in mice with experimental nutritional steatohepatitis: role as pro-inflammatory mediator. Hepatology. 2006; 43:826–836.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Obesity and binge alcohol intake are deadly combination to induce steatohepatitis: A model of high-fat diet and binge ethanol intake
- Effect of Artemisia Capillaris Extract on the Growth of Food-Borne Pathogens
- A Comparative Study of Dichloromethane and Ethyl Acetate Root Extracts of Celosia trigyna: Phytochemical and Wound Healing Effect Analyses
- Artemisia annua extract ameliorates high-fat diet-induced fatty liver by activating AMPK
- Antimicrobial Effect of Pulsatilla Koreana Extracts on Food-Borne Pathogens