J Vet Sci.
2017 Mar;18(1):11-16. 10.4142/jvs.2017.18.1.11.
Temporal changes in mammalian target of rapamycin (mTOR) and phosphorylated-mTOR expressions in the hippocampal CA1 region of rat with vascular dementia
- Affiliations
-
- 1Department of Pharmacy, College of Pharmacy, Dankook University, Cheonan 31116, Korea. anaphy@dankook.ac.kr
- KMID: 2412582
- DOI: http://doi.org/10.4142/jvs.2017.18.1.11
Abstract
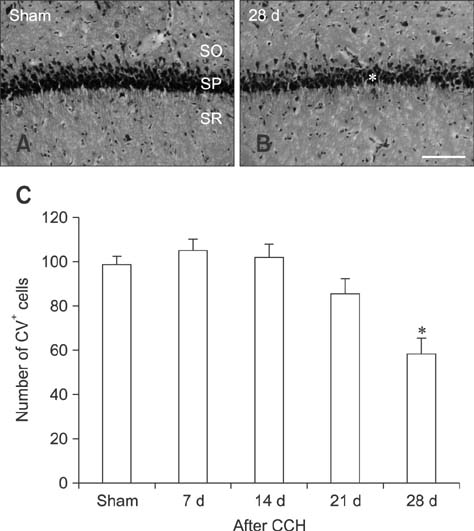
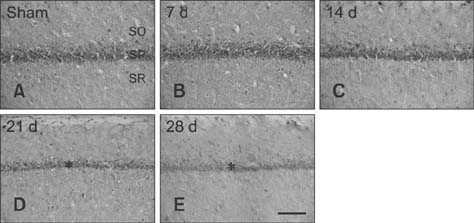
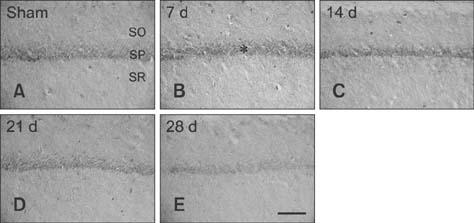
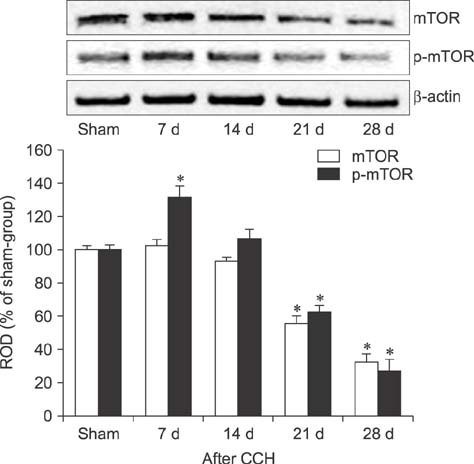
- Mammalian target of rapamycin (mTOR) has an important role in various biological processes in cells. In the present study, we investigated temporal changes in mTOR and phosphorylated-mTOR (p-mTOR) expressions in the rat hippocampal CA1 region following chronic cerebral hypoperfusion (CCH) induced by permanent bilateral common carotid arteries occlusion (2VO). The mTOR immunoreactivity in the pyramidal neurons and mTOR protein level in the hippocampal CA1 region were markedly decreased at 21 and 28 days after 2VO surgery. However, p-mTOR protein expression was significantly increased at 7 days following CCH but then decreased with time. The results indicate that mTOR and p-mTOR expressions change in the hippocampal CA1 region after 2VO surgery and that reduced expressions of mTOR and p-mTOR may be closely related to the CCH-induced neuronal damage in the hippocampal CA1 region.
Keyword
MeSH Terms
Figure
Reference
-
1. Cechetti F, Pagnussat AS, Worm PV, Elsner VR, Ben J, da Costa MS, Mestriner R, Weis SN, Netto CA. Chronic brain hypoperfusion causes early glial activation and neuronal death, and subsequent long-term memory impairment. Brain Res Bull. 2012; 87:109–116.
Article2. Chen H, Qu Y, Tang B, Xiong T, Mu D. Role of mammalian target of rapamycin in hypoxic or ischemic brain injury: potential neuroprotection and limitations. Rev Neurosci. 2012; 23:279–287.
Article3. Chong ZZ, Shang YC, Zhang L, Wang S, Maiese K. Mammalian target of rapamycin: hitting the bull's-eye for neurological disorders. Oxid Med Cell Longev. 2010; 3:374–391.
Article4. Chong ZZ, Yao Q, Li HH. The rationale of targeting mammalian target of rapamycin for ischemic stroke. Cell Signal. 2013; 25:1598–1607.
Article5. Farkas E, Donka G, de Vos RAI, Mihály A, Bari F, Luiten PGM. Experimental cerebral hypoperfusion induces white matter injury and microglial activation in the rat brain. Acta Neuropathol. 2004; 108:57–64.
Article6. Farkas E, Luiten PGM, Bari F. Permanent, bilateral common carotid artery occlusion in the rat: a model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res Rev. 2007; 54:162–180.
Article7. Fletcher L, Evans TM, Watts LT, Jimenez DF, Digicaylioglu M. Rapamycin treatment improves neuron viability in an in vitro model of stroke. PLoS One. 2013; 8:e68281.
Article8. He XL, Wang YH, Bi MG, Du GH. Chrysin improves cognitive deficits and brain damage induced by chronic cerebral hypoperfusion in rats. Eur J Pharmacol. 2012; 680:41–48.
Article9. Jia Y, Jin W, Xiao Y, Dong Y, Wang T, Fan M, Xu J, Meng N, Li L, Lv P. Lipoxin A4 methyl ester alleviates vascular cognition impairment by regulating the expression of proteins related to autophagy and ER stress in the rat hippocampus. Cell Mol Biol Lett. 2015; 20:475–487.
Article10. Kim YC, Guan KL. mTOR: a pharmacologic target for autophagy regulation. J Clin Invest. 2015; 125:25–32.
Article11. Koh PO. Melatonin prevents ischemic brain injury through activation of the mTOR/p70S6 kinase signaling pathway. Neurosci Lett. 2008; 444:74–78.
Article12. Koh PO, Cho JH, Won CK, Lee HJ, Sung JH, Kim MO. Estradiol attenuates the focal cerebral ischemic injury through mTOR/p70S6 kinase signaling pathway. Neurosci Lett. 2008; 436:62–66.
Article13. Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012; 149:274–293.
Article14. Lee CH, Ahn JH, Won MH. New expression of 5-HT1A receptor in astrocytes in the gerbil hippocampal CA1 region following transient global cerebral ischemia. Neurol Sci. 2015; 36:383–389.
Article15. Lee CH, Park JH, Yoo KY, Choi JH, Hwang IK, Ryu PD, Kim DH, Kwon YG, Kim YM, Won MH. Pre- and post-treatments with escitalopram protect against experimental ischemic neuronal damage via regulation of BDNF expression and oxidative stress. Exp Neurol. 2011; 229:450–459.
Article16. Lee CH, Yoo KY, Choi JH, Park OK, Hwang IK, Kim SK, Kang IJ, Kim YM, Won MH. Neuronal damage is much delayed and microgliosis is more severe in the aged hippocampus induced by transient cerebral ischemia compared to the adult hippocampus. J Neurol Sci. 2010; 294:1–6.
Article17. Maiese K, Chong ZZ, Shang YC, Wang S. mTOR: on target for novel therapeutic strategies in the nervous system. Trends Mol Med. 2013; 19:51–60.
Article18. Ozacmak VH, Barut F, Ozacmak HS. Melatonin provides neuroprotection by reducing oxidative stress and HSP70 expression during chronic cerebral hypoperfusion in ovariectomized rats. J Pineal Res. 2009; 47:156–163.
Article19. Park JA, Lee CH. Time-course change of Redd1 expressions in the hippocampal CA1 region following chronic cerebral hypoperfusion. Cell Mol Neurobiol. 2016; 37:563–569. DOI: 10.1007/s10571-016-0385-9.
Article20. Román GC. Brain hypoperfusion: a critical factor in vascular dementia. Neurol Res. 2004; 26:454–458.
Article21. Shi GD, OuYang YP, Shi JG, Liu Y, Yuan W, Jia LS. PTEN deletion prevents ischemic brain injury by activating the mTOR signaling pathway. Biochem Biophys Res Commun. 2011; 404:941–945.
Article22. Ueno Y, Koike M, Shimada Y, Shimura H, Hira K, Tanaka R, Uchiyama Y, Hattori N, Urabe T. L-carnitine enhances axonal plasticity and improves white-matter lesions after chronic hypoperfusion in rat brain. J Cereb Blood Flow Metab. 2015; 35:382–391.
Article23. Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006; 124:471–484.
Article24. Yang H, Shi O, Jin Y, Henrich-Noack P, Qiao H, Cai C, Tao H, Tian X. Functional protection of learning and memory abilities in rats with vascular dementia. Restor Neurol Neurosci. 2014; 32:689–700.
Article25. Yang X, Hei C, Liu P, Song Y, Thomas T, Tshimanga S, Wang F, Niu J, Sun T, Li PA. Inhibition of mTOR pathway by rapamycin reduces brain damage in rats subjected to transient forebrain ischemia. Int J Biol Sci. 2015; 11:1424–1435.
Article26. Yin L, Ye S, Chen Z, Zeng Y. Rapamycin preconditioning attenuates transient focal cerebral ischemia/reperfusion injury in mice. Int J Neurosci. 2012; 122:748–756.
Article27. Yu L, McPhee CK, Zheng L, Mardones GA, Rong Y, Peng J, Mi N, Zhao Y, Liu Z, Wan F, Hailey DW, Oorschot V, Klumperman J, Baehrecke EH, Lenardo MJ. Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature. 2010; 465:942–946.
Article28. Zare Mehrjerdi F, Aboutaleb N, Habibey R, Ajami M, Soleimani M, Arabian M, Niknazar S, Hossein Davoodi S, Pazoki-Toroudi H. Increased phosphorylation of mTOR is involved in remote ischemic preconditioning of hippocampus in mice. Brain Res. 2013; 1526:94–101.
Article29. Zhu Y, Zeng Y, Wang X, Ye X. Effect of electroacupuncture on the expression of mTOR and eIF4E in hippocampus of rats with vascular dementia. Neurol Sci. 2013; 34:1093–1097.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neuroprotective Effect of Duloxetine on Chronic Cerebral Hypoperfusion-Induced Hippocampal Neuronal Damage
- Where is the Mechanistic Target of Rapamycin Signaling Pathway in Depression?
- Mammalian Target of Rapamycin Signaling Pathways and Depression
- The role of amino acid-induced mammalian target of rapamycin complex 1(mTORC1) signaling in insulin resistance
- Long and Short-Term Effect of mTOR Regulation on Cerebral Organoid Growth and Differentiations