J Vet Sci.
2017 Sep;18(3):359-367. 10.4142/jvs.2017.18.3.359.
Evaluation of expression of the Wnt signaling components in canine mammary tumors via RT² Profiler PCR Array and immunochemistry assays
- Affiliations
-
- 1Department of Veterinary Clinical Science, College of Veterinary Medicine, China Agricultural University, Beijing 100193, China. csama@sina.com
- 2Clinical Pathology Department, Dick White Referrals, Cambridgeshire, CB8 0UH, UK.
- KMID: 2412454
- DOI: http://doi.org/10.4142/jvs.2017.18.3.359
Abstract
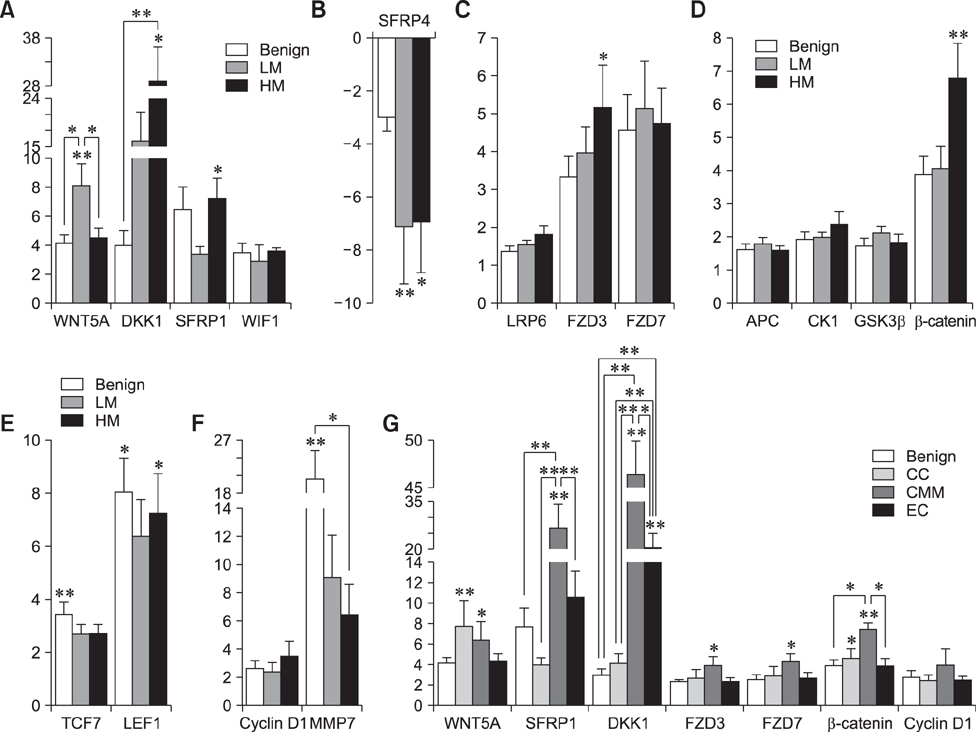
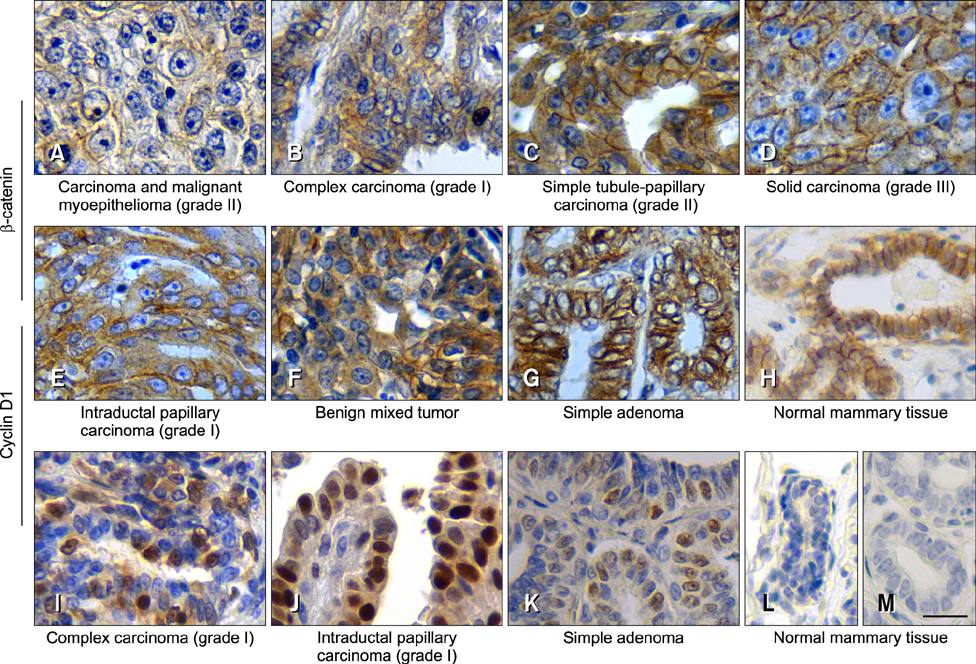
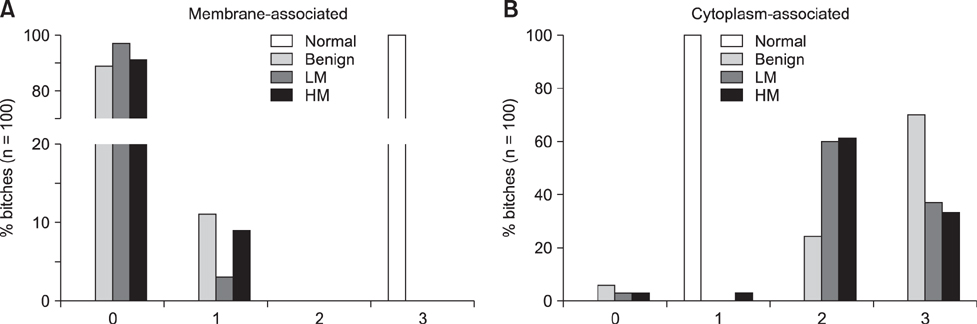
- The Wnt signaling pathway and its key component β-catenin have critical roles in the development of diseases such as tumors in mammals. However, little has been reported about involvement of the Wnt/β-catenin signaling pathway in canine mammary tumors (CMTs). The present study detected expression of 30 Wnt signaling pathway-related genes in CMTs; the results are potentially useful for molecular-based diagnosis of CMTs and the development of new targeted therapies. Significant upregulations of dickkopf-1 protein, secreted frizzled-related sequence protein 1 (SFRP1), frizzled 3, β-catenin, and lymphoid enhancer-binding factor 1 (LEF1) were detected in highly malignant CMTs compared to levels in normal mammary gland tissues; moreover, highly significant upregulation of WNT5A was observed in low malignancy CMTs. Downregulation was only detected for SFRP4 in malignant CMT samples. The subcellular location of β-catenin and cyclin D1 in 100 CMT samples was investigated via immunohistochemical analysis, and significantly increased expressions of β-catenin in cytoplasm and cyclin D1 in nuclei were revealed. Western blotting analysis revealed that the expression of β-catenin and LEF1 increased in in the majority of CMT samples. Taken together, the results provide important evidence of the activation status of the Wnt pathway in CMTs and valuable clues to identifying biomarkers for molecular-based diagnosis of CMT.
MeSH Terms
-
Animals
Blotting, Western/veterinary
Cyclin D1/metabolism
Dog Diseases/*metabolism/pathology
Dogs
Female
Gene Expression Regulation, Neoplastic
Mammary Neoplasms, Animal/*metabolism/pathology
Polymerase Chain Reaction/veterinary
Retrospective Studies
*Wnt Signaling Pathway
Wnt-5a Protein/metabolism
beta Catenin/metabolism
Wnt-5a Protein
beta Catenin
Cyclin D1
Figure
Reference
-
1. Brunetti B, Sarli G, Preziosi R, Monari I, Benazzi C. E-cadherin and β-catenin reduction influence invasion but not proliferation and survival in canine malignant mammary tumors. Vet Pathol. 2005; 42:781–787.
Article2. Clevers H. Wnt/β-catenin signaling in development and disease. Cell. 2006; 127:469–480.
Article3. Cuello-Carrión FD, Shortrede JE, Alvarez-Olmedo D, Cayado-Gutiérrez N, Castro GN, Zoppino FCM, Guerrero M, Martinis E, Wuilloud R, Gómez NN, Biaggio V, Orozco J, Gago FE, Ciocca LA, Fanelli MA, Ciocca DR. HER2 and β-catenin protein location: importance in the prognosis of breast cancer patients and their correlation when breast cancer cells suffer stressful situations. Clin Exp Metastasis. 2015; 32:151–168.
Article4. Gama A, Paredes J, Gärtner F, Alves A, Schmitt F. Expression of E-cadherin, P-cadherin and β-catenin in canine malignant mammary tumours in relation to clinicopathological parameters, proliferation and survival. Vet J. 2008; 177:45–53.
Article5. Goldschmidt M, Peña L, Rasotto R, Zappulli V. Classification and grading of canine mammary tumors. Vet Pathol. 2011; 48:117–131.
Article6. Gracanin A, Timmermans-Sprang EPM, van Wolferen ME, Rao NAS, Grizelj J, Vince S, Hellmen E, Mol JA. Ligand-independent canonical Wnt activity in canine mammary tumor cell lines associated with aberrant LEF1 expression. PLoS One. 2014; 9:e98698.
Article7. Han SX, Zhou X, Sui X, He CC, Cai MJ, Ma JL, Zhang YY, Zhou CY, Ma CX, Varela-Ramirez A, Zhu Q. Serum dickkopf-1 is a novel serological biomarker for the diagnosis and prognosis of pancreatic cancer. Oncotarget. 2015; 6:19907–19917.
Article8. Im KS, Kim NH, Lim HY, Kim HW, Shin JI, Sur JH. Analysis of a new histological and molecular-based classification of canine mammary neoplasia. Vet Pathol. 2014; 51:549–559.
Article9. Kam Y, Quaranta V. Cadherin-bound β-catenin feeds into the Wnt pathway upon adherens junctions dissociation: evidence for an intersection between β-catenin pools. PLoS One. 2009; 4:e4580.
Article10. Kang CM, Kim HK, Kim H, Choi GH, Kim KS, Choi JS, Lee WJ. Expression of Wnt target genes in solid pseudopapillary tumor of the pancreas: a pilot study. Pancreas. 2009; 38:e53–e59.11. Khramtsov AI, Khramtsova GF, Tretiakova M, Huo D, Olopade OI, Goss KH. Wnt/β-catenin pathway activation is enriched in basal-like breast cancers and predicts poor outcome. Am J Pathol. 2010; 176:2911–2920.
Article12. Kipp A, Banning A, van Schothorst EM, Méplan C, Schomburg L, Evelo C, Coort S, Gaj S, Keijer J, Hesketh J, Brigelius-Flohé R. Four selenoproteins, protein biosynthesis, and Wnt signalling are particularly sensitive to limited selenium intake in mouse colon. Mol Nutr Food Res. 2009; 53:1561–1572.
Article13. Li S, Li S, Sun Y, Li L. The expression of β-catenin in different subtypes of breast cancer and its clinical significance. Tumour Biol. 2014; 35:7693–7698.
Article14. Lim SC, Lee MS. Significance of E-cadherin/β-catenin complex and cyclin D1 in breast cancer. Oncol Rep. 2002; 9:915–928.
Article15. Loh YN, Hedditch EL, Baker LA, Jary E, Ward RL, Ford CE. The Wnt signalling pathway is upregulated in an in vitro model of acquired tamoxifen resistant breast cancer. BMC Cancer. 2013; 13:174.
Article16. MacDonald BT, Tamai K, He X. Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009; 17:9–26.
Article17. Murakami Y, Tateyama S, Rungsipipat A, Uchida K, Yamaguchi R. Immunohistochemical analysis of cyclin A, cyclin D1 and P53 in mammary tumors, squamous cell carcinomas and basal cell tumors of dogs and cats. J Vet Med Sci. 2000; 62:743–750.
Article18. Peña L, De Andrés PJ, Clemente M, Cuesta P, Pérez-Alenza MD. Prognostic value of histological grading in noninflammatory canine mammary carcinomas in a prospective study with two-year follow-up: relationship with clinical and histological characteristics. Vet Pathol. 2013; 50:94–105.
Article19. Polakis P. The many ways of Wnt in cancer. Curr Opin Genet Dev. 2007; 17:45–51.
Article20. Queiroga FL, Raposo T, Carvalho MI, Prada J, Pires I. Canine mammary tumours as a model to study human breast cancer: most recent findings. In Vivo. 2011; 25:455–465.21. Rasotto R, Goldschmidt MH, Castagnaro M, Carnier P, Caliari D, Zappulli V. The dog as a natural animal model for study of the mammary myoepithelial basal cell lineage and its role in mammary carcinogenesis. J Comp Pathol. 2014; 151:166–180.
Article22. Rasotto R, Zappulli V, Castagnaro M, Goldschmidt MH. A retrospective study of those histopathologic parameters predictive of invasion of the lymphatic system by canine mammary carcinomas. Vet Pathol. 2012; 49:330–340.
Article23. Ren XY, Zhou GQ, Jiang W, Sun Y, Xu YF, Li YQ, Tang XR, Wen X, He QM, Yang XJ, Liu N, Ma J. Low SFRP1 expression correlates with poor prognosis and promotes cell invasion by activating the Wnt/β-catenin signaling pathway in NPC. Cancer Prev Res (Phila). 2015; 8:968–977.
Article24. Restucci B, Maiolino P, Martano M, Esposito G, De Filippis D, Borzacchiello G, Lo Muzio L. Expression of β-catenin, E-cadherin and APC in canine mammary tumors. Anticancer Res. 2007; 27:3083–3089.25. Rowell JL, McCarthy DO, Alvarez CE. Dog models of naturally occurring cancer. Trends Mol Med. 2011; 17:380–388.
Article26. Shen Q, Fan J, Yang XR, Tan Y, Zhao W, Xu Y, Wang N, Niu Y, Wu Z, Zhou J, Qiu SJ, Shi YH, Yu B, Tang N, Chu W, Wang M, Wu J, Zhang Z, Yang S, Gu J, Wang H, Qin W. Serum DKK1 as a protein biomarker for the diagnosis of hepatocellular carcinoma: a large-scale, multicentre study. Lancet Oncol. 2012; 13:817–826.
Article27. Sorenmo K. Canine mammary gland tumors. Vet Clin North Am Small Anim Pract. 2003; 33:573–596.
Article28. Uva P, Aurisicchio L, Watters J, Loboda A, Kulkarni A, Castle J, Palombo F, Viti V, Mesiti G, Zappulli V, Marconato L, Abramo F, Ciliberto G, Lahm A, La Monica N, de Rinaldis E. Comparative expression pathway analysis of human and canine mammary tumors. BMC Genomics. 2009; 10:135.
Article29. Valenta T, Hausmann G, Basler K. The many faces and functions of β-catenin. EMBO J. 2012; 31:2714–2736.
Article30. Yao X, Jiang H, Zhang C, Wang H, Yang L, Yu Y, Yu J, Shi B, Shen Z, Gao H, Chen Z, Tian S, Lu S, Li Z, Gu J. Dickkopf-1 autoantibody is a novel serological biomarker for non-small cell lung cancer. Biomarkers. 2010; 15:128–134.
Article31. Zheng L, Sun D, Fan W, Zhang Z, Li Q, Jiang T. Diagnostic value of SFRP1 as a favorable predictive and prognostic biomarker in patients with prostate cancer. PLoS One. 2015; 10:e0118276.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Alpha basic crystallin expression in canine mammary tumors
- Expression of CD133, CD44, CK7, and OCT4 in Animal Cancers
- Natural Products Targeting Wnt/β-catenin Signaling Pathway
- Mechanisms of the Wnt Pathways as a Potential Target Pathway in Atherosclerosis
- Mutations of p53 Tumor Suppressor Gene in Spontaneous Canine Mammary Tumors