J Adv Prosthodont.
2018 Apr;10(2):147-154. 10.4047/jap.2018.10.2.147.
Analysis of osteogenic potential on 3mol% yttria-stabilized tetragonal zirconia polycrystals and two different niobium oxide containing zirconia ceramics
- Affiliations
-
- 1Department of Prosthodontics, School of Dentistry, Seoul National University, Seoul, Republic of Korea. proshan@snu.ac.kr
- 2Department of Periodontology, School of Dentistry and Dental Research Institute, BK21 Program, Seoul National University, Seoul, Republic of Korea.
- 3Department of Advanced Materials Engineering, Sejong University, Seoul, Republic of Korea.
- KMID: 2409635
- DOI: http://doi.org/10.4047/jap.2018.10.2.147
Abstract
- PURPOSE
This study was performed to evaluate the osteogenic potential of 3mol% yttria-stabilized tetragonal zirconia polycrystals (3Y-TZP) and niobium oxide containing Y-TZPs with specific ratios, new (Y,Nb)-TZPs, namely YN4533 and YN4533/Al20 discs.
MATERIALS AND METHODS
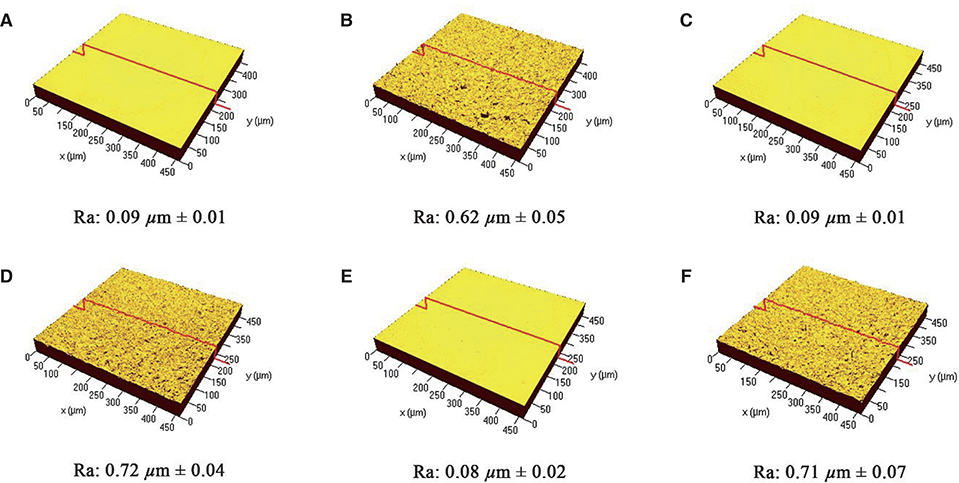
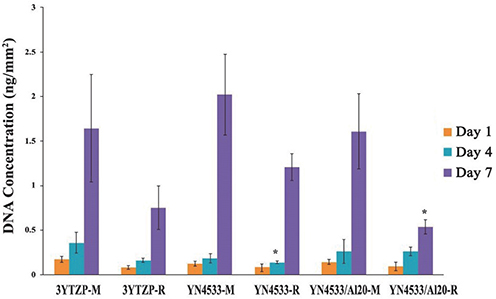
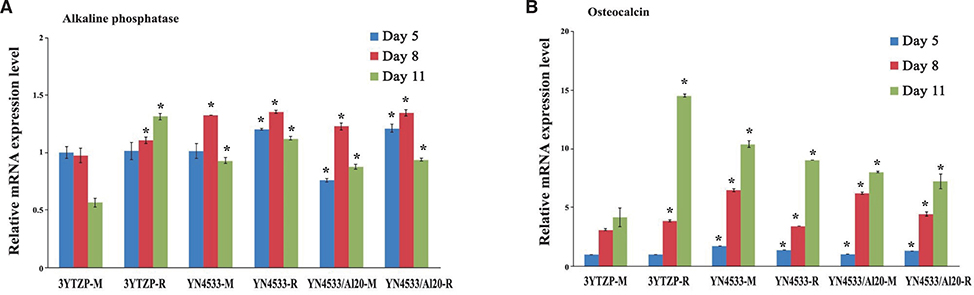
3Y-TZP, YN4533 and YN4533/Al20 discs (15 mm diameter and 1 mm thickness) were prepared and their average surface roughness (Ra) and surface topography were analyzed using 3-D confocal laser microscope (CLSM) and scanning electron microscope (SEM). Mouse pre-osteoblast MC3T3-E1 cells were seeded onto all zirconia discs and evaluated with regard to cell attachment and morphology by (CLSM), cell proliferation by PicoGreen assay, and cell differentiation by Reverse-Transcription PCR and Quantitative Real-Time PCR, and alkaline phosphatase (Alp) staining.
RESULTS
The cellular morphology of MC3T3-E1 pre-osteoblasts was more stretched on a smooth surface than on a rough surface, regardless of the material. Cellular proliferation was higher on smooth surfaces, but there were no significant differences between 3Y-TZP, YN4533, and YN4533/Al20. Osteoblast differentiation patterns on YN4533 and YN4533/Al20 were similar to or slightly higher than seen in 3Y-TZP. Although there were no significant differences in bone marker gene expression (alkaline phosphatase and osteocalcin), Alp staining indicated better osteoblast differentiation on YN4533 and YN4533/Al20 compared to 3Y-TZP.
CONCLUSION
Based on these results, niobium oxide containing Y-TZPs have comparable osteogenic potential to 3Y-TZP and are expected to be suitable alternative ceramics dental implant materials to titanium for aesthetically important areas.
MeSH Terms
Figure
Reference
-
1. Astrand P, Ahlqvist J, Gunne J, Nilson H. Implant treatment of patients with edentulous jaws: a 20-year follow-up. Clin Implant Dent Relat Res. 2008; 10:207–217.2. Adell R, Eriksson B, Lekholm U, Brånemark PI, Jemt T. Long-term follow-up study of osseointegrated implants in the treatment of totally edentulous jaws. Int J Oral Maxillofac Implants. 1990; 5:347–359.3. Steinemann SG. Titanium-the material of choice? Periodontol 2000. 1998; 17:7–21.4. Depprich R, Zipprich H, Ommerborn M, Naujoks C, Wiesmann HP, Kiattavorncharoen S, Lauer HC, Meyer U, Kübler NR, Handschel J. Osseointegration of zirconia implants compared with titanium: an in vivo study. Head Face Med. 2008; 4:30.
Article5. Heydecke G, Kohal R, Gläser R. Optimal esthetics in single-tooth replacement with the Re-Implant system: a case report. Int J Prosthodont. 1999; 12:184–189.6. Sailer I, Zembic A, Jung RE, Hämmerle CH, Mattiola A. Single-tooth implant reconstructions: esthetic factors influencing the decision between titanium and zirconia abutments in anterior regions. Eur J Esthet Dent. 2007; 2:296–310.7. Sicilia A, Cuesta S, Coma G, Arregui I, Guisasola C, Ruiz E, Maestro A. Titanium allergy in dental implant patients: a clinical study on 1500 consecutive patients. Clin Oral Implants Res. 2008; 19:823–835.
Article8. Siddiqi A, Payne AG, De Silva RK, Duncan WJ. Titanium allergy: could it affect dental implant integration? Clin Oral Implants Res. 2011; 22:673–680.
Article9. Nakagawa M, Matsuya S, Udoh K. Effects of fluoride and dissolved oxygen concentrations on the corrosion behavior of pure titanium and titanium alloys. Dent Mater J. 2002; 21:83–92.
Article10. Tschernitschek H, Borchers L, Geurtsen W. Nonalloyed titanium as a bioinert metal-a review. Quintessence Int. 2005; 36:523–530.
Article11. Osman RB, Swain MV. A Critical review of dental implant materials with an emphasis on titanium versus zirconia. Materials (Basel). 2015; 8:932–958.12. Piconi C, Maccauro G. Zirconia as a ceramic biomaterial. Biomaterials. 1999; 20:1–25.
Article13. Zarone F, Russo S, Sorrentino R. From porcelain-fused-to-metal to zirconia: clinical and experimental considerations. Dent Mater. 2011; 27:83–96.
Article14. Zembic A, Bösch A, Jung RE, Hämmerle CH, Sailer I. Fiveyear results of a randomized controlled clinical trial comparing zirconia and titanium abutments supporting single-implant crowns in canine and posterior regions. Clin Oral Implants Res. 2013; 24:384–390.
Article15. Wenz HJ, Bartsch J, Wolfart S, Kern M. Osseointegration and clinical success of zirconia dental implants: a systematic re-view. Int J Prosthodont. 2008; 21:27–36.16. Manzano G, Herrero LR, Montero J. Comparison of clinical performance of zirconia implants and titanium implants in animal models: a systematic review. Int J Oral Maxillofac Implants. 2014; 29:311–320.
Article17. Cionca N, Hashim D, Mombelli A. Zirconia dental implants: where are we now, and where are we heading? Periodontol 2000. 2017; 73:241–258.
Article18. Bosshardt DD, Chappuis V, Buser D. Osseointegration of titanium, titanium alloy and zirconia dental implants: current knowledge and open questions. Periodontol 2000. 2017; 73:22–40.
Article19. Bächle M, Butz F, Hübner U, Bakalinis E, Kohal RJ. Behavior of CAL72 osteoblast-like cells cultured on zirconia ceramics with different surface topographies. Clin Oral Implants Res. 2007; 18:53–59.
Article20. Hempel U, Hefti T, Kalbacova M, Wolf-Brandstetter C, Dieter P, Schlottig F. Response of osteoblast-like SAOS-2 cells to zirconia ceramics with different surface topographies. Clin Oral Implants Res. 2010; 21:174–181.
Article21. Scarano A, Di Carlo F, Quaranta M, Piattelli A. Bone response to zirconia ceramic implants: an experimental study in rabbits. J Oral Implantol. 2003; 29:8–12.
Article22. Hoffmann O, Angelov N, Gallez F, Jung RE, Weber FE. The zirconia implant-bone interface: a preliminary histologic evaluation in rabbits. Int J Oral Maxillofac Implants. 2008; 23:691–695.23. Gahlert M, Roehling S, Sprecher CM, Kniha H, Milz S, Bormann K. In vivo performance of zirconia and titanium implants: a histomorphometric study in mini pig maxillae. Clin Oral Implants Res. 2012; 23:281–286.
Article24. Kim DJ, Jung HJ, Cho DH. Phase transformations of Y2O3 and Nb2O5 doped tetragonal zirconia during low temperature aging in air. Solid State Ion. 1995; 80:67–73.
Article25. Kelly JR, Denry I. Stabilized zirconia as a structural ceramic: an overview. Dent Mater. 2008; 24:289–298.
Article26. Nawa M, Nakamoto S, Sekino T, Niihara K. Tough and strong Ce-TZP/alumina nanocomposites doped with titania. Ceram Int. 1998; 24:497–506.
Article27. Takano T, Tasaka A, Yoshinari M, Sakurai K. Fatigue strength of Ce-TZP/Al2O3 nanocomposite with different surfaces. J Dent Res. 2012; 91:800–804.
Article28. Andreiotelli M, Kohal RJ. Fracture strength of zirconia implants after artificial aging. Clin Implant Dent Relat Res. 2009; 11:158–166.
Article29. Kohal RJ, Wolkewitz M, Mueller C. Alumina-reinforced zirconia implants: survival rate and fracture strength in a masticatory simulation trial. Clin Oral Implants Res. 2010; 21:1345–1352.
Article30. Chappuis V, Cavusoglu Y, Gruber R, Kuchler U, Buser D, Bosshardt DD. Osseointegration of zirconia in the presence of multinucleated giant cells. Clin Implant Dent Relat Res. 2016; 18:686–698.
Article31. Kim DJ, Jung HJ, Jang JW, Lee HL. Fracture toughness, ionic conductivity, and low-temperature phase stability of tetragonal zirconia codoped with Yttria and Niobium Oxide. J Am Ceram Soc. 1998; 81:2309–2314.
Article32. Ray JC, Panda AB, Saha CR, Pramanik P. Synthesis of niobium(V)- stabilized tetragonal zirconia nanocrystalline powders. J Am Ceram Soc. 2003; 86:514–516.33. Gutiérrez-González CF, Moya JS, Palomares FJ, Bartolomé Gómez JF. Low-temperature aging degradation-free 3Y-TZP/ Nb composites. J Am Ceram Soc. 2010; 93:1842–1844.34. Li P, Chen IW, Penner-Hahn JE. Effect of dopants on zirconia stabilization - An X-ray absorption study: III, charge-compensating dopants. J Am Ceram Soc. 1994; 77:1289–1295.35. Guo X, Wang Z. Effect of niobia on the defect structure of yttria-stabilized zirconia. J Euro Ceram Soc. 1998; 18:237–240.
Article36. Cho YD, Shin JC, Kim HL, Gerelmaa M, Yoon HI, Ryoo HM, Kim DJ, Han JS. Comparison of the osteogenic potential of titanium- and modified zirconia-based bioceramics. Int J Mol Sci. 2014; 15:4442–4452.
Article37. Johansson CB, Albrektsson T. A removal torque and histomorphometric study of commercially pure niobium and titanium implants in rabbit bone. Clin Oral Implants Res. 1991; 2:24–29.
Article38. Matsuno H, Yokoyama A, Watari F, Uo M, Kawasaki T. Biocompatibility and osteogenesis of refractory metal implants, titanium, hafnium, niobium, tantalum and rhenium. Biomaterials. 2001; 22:1253–1262.
Article39. Godley R, Starosvetsky D, Gotman I. Bonelike apatite formation on niobium metal treated in aqueous NaOH. J Mater Sci Mater Med. 2004; 15:1073–1077.
Article40. Kim DJ. Effect of Ta2O5, Nb2O5, and HfO2 alloying on the transformability of Y2O3-stabilized tetragonal ZrO2. J Am Ceram Soc. 1990; 73:115–120.
Article41. Kim DJ, Lee MH, Lee DY, Han JS. Mechanical properties, phase stability, and biocompatibility of (Y,Nb)-TZP/Al2O3 composite abutments for dental implant. J Biomed Mater Res. 2000; 53:438–443.
Article42. Ewais O, Al Abbassy F, Ghoneim MM, Aboushelib MN. Novel zirconia surface treatments for enhanced osseointegration: Laboratory characterization. Int J Dent. 2014; 2014:203940.
Article43. Albrektsson T, Wennerberg A. Oral implant surfaces: Part 1--review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int J Prosthodont. 2004; 17:536–543.44. Quirynen M, Van Assche N. RCT comparing minimally with moderately rough implants. Part 2: microbial observations. Clin Oral Implants Res. 2012; 23:625–634.
Article45. Taniguchi Y, Kakura K, Yamamoto K, Kido H, Yamazaki J. Accelerated Osteogenic Differentiation and Bone Formation on Zirconia with Surface Grooves Created with Fiber Laser Irradiation. Clin Implant Dent Relat Res. 2016; 18:883–894.
Article46. Raigrodski AJ, Chiche GJ, Potiket N, Hochstedler JL, Mohamed SE, Billiot S, Mercante DE. The efficacy of posterior three-unit zirconium-oxide-based ceramic fixed partial dental prostheses: a prospective clinical pilot study. J Prosthet Dent. 2006; 96:237–244.
Article47. Sailer I, Pjetursson BE, Zwahlen M, Hämmerle CH. A systematic review of the survival and complication rates of allceramic and metal-ceramic reconstructions after an observation period of at least 3 years. Part II: Fixed dental prostheses. Clin Oral Implants Res. 2007; 18 Suppl 3. 86–96.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Review on factors affecting the optical properties of dental zirconia
- Cytotoxicity and biocompatibility of high mol% yttria containing zirconia
- Properties of translucent zirconia and lithium disilicate glass-ceramics: a literature review
- Effects of Light-Curing on the Immediate and Delayed Micro-Shear Bond Strength between Yttria-Tetragonal Zirconia Polycrystal Ceramics and Universal Adhesive
- The effect of zirconia surface architecturing technique on the zirconia/veneer interfacial bond strength