Nat Prod Sci.
2018 Mar;24(1):28-35. 10.20307/nps.2018.24.1.28.
Pulegone Exhibits Anti-inflammatory Activities through the Regulation of NF-κB and Nrf-2 Signaling Pathways in LPS-stimulated RAW 264.7 cells
- Affiliations
-
- 1Department of Food and Life Science, Pukyong National University, Busan 608-737, Republic of Korea. choijs@pknu.ac.kr
- 2Department of Pharmaceutical Engineering, Sangji University, Wonju 220-702, Republic of Korea.
- 3Department of Food Science and Human Nutrition, Chonbuk National University, Jeonju 561-756, Republic of Korea. jungha@jbnu.ac.kr
- KMID: 2409606
- DOI: http://doi.org/10.20307/nps.2018.24.1.28
Abstract
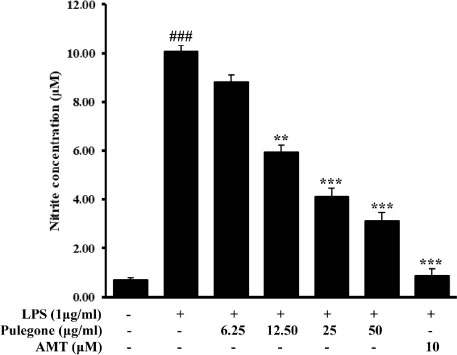
- Pulegone is a naturally occurring organic compound obtained from essential oils from a variety of plants. The aim of this study was to investigate the anti-inflammatory effects through the inhibitory mechanism of inducible nitric oxide synthase (iNOS), cyclooxygenase (COX-2), nuclear factor kappa B (NF-κB), mitogen-activated protein kinases (MAPK) pathways and the activation of nuclear factor erythroid 2-related factor 2 (Nrf2)/ heme oxygenase (HO)-1 pathways in lipopolysaccharide (LPS)-stimulated RAW264.7 cells. Results revealed that pulegone significantly inhibited NO production as well as iNOS and COX-2 expressions. Meanwhile, western blot analysis showed that pulegone down-regulated LPS-induced NF-κB and MAPKs activation in RAW 264.7 cells. Furthermore, the selected compound suppressed LPS-induced intracellular ROS production in RAW 264.7 cells, while the expression of stress response gene, HO-1, and its transcriptional activator, Nrf-2 was upregulated upon pulegone treatment. Taking together, these findings provided that pulegone inhibited the LPS-induced expression of inflammatory mediators via the down-regulation iNOS, COX-2, NF-κB, and MAPKs signaling pathways as well as up-regulation of Nrf-2/HO-1 indicating that pulegone has a potential therapeutic and preventive application in various inflammatory diseases.
Keyword
MeSH Terms
-
Blotting, Western
Down-Regulation
Heme Oxygenase (Decyclizing)
Mitogen-Activated Protein Kinases
NF-kappa B
Nitric Oxide Synthase Type II
Oils, Volatile
Prostaglandin-Endoperoxide Synthases
RAW 264.7 Cells*
Up-Regulation
Heme Oxygenase (Decyclizing)
Mitogen-Activated Protein Kinases
NF-kappa B
Nitric Oxide Synthase Type II
Oils, Volatile
Prostaglandin-Endoperoxide Synthases
Figure
Reference
-
1. Choudhari AS, Raina P, Deshpande MM, Wali AG, Zanwar A, Bodhankar SL, Kaul-Ghanekar R. J Ethnopharmacol. 2013; 150:215–222.2. Joung EJ, Lee B, Gwon WG, Shin T, Jung BM, Yoon NY, Choi JS, Oh CW, Kim HR. Int Immunopharmacol. 2015; 29:693–700.3. Giuliani C, Napolitano G, Bucci I, Montani V, Monaco F. Clin Ter. 2001; 152:249–253.4. May MJ, Ghosh S. Immunol Today. 1998; 19:80–88.5. Tak PP, Firestein GS. J Clin Invest. 2001; 107:7–11.6. Kim AR, Lee MS, Shin TS, Hua H, Jang BC, Choi JS, Byun DS, Utsuki T, Ingram D, Kim HR. Toxicol in Vitro. 2011; 25:1789–1795.7. Pae HO, Chung HT. Immune Netw. 2009; 9:12–19.8. Lee IS, Lim J, Gal J, Kang JC, Kim HJ, Kang BY, Choi HJ. Neurochem Int. 2011; 58:153–160.9. Taha R, Blaise G. Funct Food Health Dis. 2014; 4:510–523.10. Bakkali F, Averbeck S, Averbeck D, Idaomar M. Food Chem Toxicol. 2008; 46:446–475.11. Sullivan JB, Rumack BH, Thomas H, Peterson RG, Bryson P. J Am Med Assoc. 1979; 242:2873–2874.12. Choi JS, Song BM, Park HJ. Kor J Pharmacogn. 2016; 47:192–196.13. Kumar P, Mishra S, Malik A, Satya S. Ind Crops Prod. 2011; 34:802–817.14. Brahmi F, Abdenour A, Bruno M, Silvia P, Alessandra P, Danilo F, Drifa YG, Fahmi EM, Khodir M, Mohamed C. Ind Crops Prod. 2016; 88:96–105.15. Ertas A, Gören AC, Hasimi N, Tolan V, Kolak U. Rec Nat Prod. 2015; 9:105–115.16. Jung HA, Roy A, Abdul QA, Kim HR, Park HJ, Choi JS. Nat Prod Sci. 2017; 23:183–191.17. Yim VWC, Ng AKY, Tsang HWH, Leung AY. J Altern Complement Med. 2009; 15:187–195.18. Moss M, Hewitt S, Moss L, Wesnes K. Int J Neurosci. 2008; 118:59–77.19. Nath SS, Pandey C, Roy D. Indian J Anaesth. 2012; 56:582–584.20. Di Stasi LC, Oliveira GP, Carvalhaes MA, Queiroz-Junior M, Tien OS, Kakinami SH, Reis MS. Fitoterapia. 2002; 73:69–91.21. Beikmohammadi M. World Appl Sci J. 2011; 12:1635–1638.22. McClanahan RH, Thomassen D, Slattery JT, Nelson SD. Chem Res Toxicol. 1989; 2:349–355.23. Bakerink JA, Gospe SM Jr, Dimand RJ, Eldridge MW. Pediatrics. 1996; 98:944–947.24. de Sousa DP, Nóbrega FFF, de Lima MRV, de Almeida RN. Naturforsch C. 2011; 66:353–359.25. Umezu T. Pharmacol Biochem Behav. 2010; 94:497–502.26. Yao QS, Chiou GC. Zhongguo Yao Li Xue Bao. 1993; 14:13–17.27. Wen TQ, Sang WT, Xu F, Wang F, Zeng N. Zhongguo Zhong Yao Za Zhi. 2016; 41:4642–4647.28. Meda L, Cassatella MA, Szendrei GI, Otvos L Jr, Baron P, Villalba M, Ferrari D, Rossi F. Nature. 1995; 374:647–650.29. Dandona P, Chaudhuri A, Dhindsa S. Diabetes Care. 2010; 33:1686–1687.30. Marks-Konczalik J, Chu SC, Moss J. J Biol Chem. 1998; 273:22201–22208.31. Islam MN, Choi RJ, Jin SE, Kim YS, Ahn BR, Zhao D, Jung HA, Choi JS. J Ethnopharmacol. 2012; 144:175–181.32. Chen JJ, Huang WC, Chen CC. Mol Biol Cell. 2005; 16:5579–5591.33. Kaminska B. Biochim Biophys Acta. 2005; 1754:253–262.34. Hancock JT, Desikan R, Neill S. J Biochem Soc Trans. 2001; 29:345–349.35. Choi SY, Hwang JH, Ko HC, Park JG, Kim SJ. J Ethnopharmacol. 2007; 113:149–155.36. Siomek A. Acta Biochem Pol. 2012; 59:323–331.37. Ryan KA, Smith MF Jr, Sanders MK, Ernst PB. Infect Immun. 2004; 72:2123–2130.38. Kim JH, Choo YY, Tae N, Min BS, Lee JH. Int Immunopharmacol. 2014; 22:420–426.39. Lee MY, Lee JA, Seo CS, Ha H, Lee H, Son JK, Shin HK. Food Chem Toxicol. 2011; 49:1047–1055.40. Paine A, Eiz-Vesper B, Blasczyk R, Immenschuh S. Biochem Pharmacol. 2010; 80:1895–1903.41. Tsoyi K, Lee TY, Lee YS, Kim HJ, Seo HG, Lee JH, Chang KC. Mol Pharmacol. 2009; 76:173–182.42. Auerbach SS, Elmore SA, Bishop JB, Bucher JR, Chan PC, Chhabra RS, Foster PM, Herbert RA, Hooth MJ, King-Herbert AP, Kissling GE, Malarkey DE, Roycroft JH, Sanders JM, Smith CS, Travlos GS, Walker NJ, Witt KL, Hejtmancik MR, Sells DM, Skowronek AJ, Toft JD II, Hamlin MH II, Brix AE, Hard GC, Kolenda-Roberts HM, Peckham JC, Wolfe GW, Seung HS, Brecher S, Iyer S, Tharakan VS, Morrison JP, Elmore SA, Flake GP, Hard GC, Kolenda-Roberts HM, Malarkey DE, Maronpot RR, Peckham JC, Morrison JP, Belpoggi F, Brix AE, Elmore SA, Flake GP, Hard GC, Herbert RA, Malarkey DE, Maronpot RR, Wakamatsu N, Crockett PW, Betz LJ, McGowan KP, Gunnels SR, Coker KK, Harper LM, Serbus DC. Natl Toxicol Program Tech Rep Ser. 2011; 563:1–201.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Estragole Exhibits Anti-inflammatory Activity with the Regulation of NF-κB and Nrf-2 Signaling Pathways in LPS-induced RAW 264.7 cells
- Anti-inflammatory activities of Scolopendra subspinipes mutilans in RAW 264.7 cells
- Luteolin 5-O-glucoside from Korean Milk Thistle, Cirsium maackii, Exhibits Anti-Inflammatory Activity via Activation of the Nrf2/HO-1 Pathway
- Anti-inflammatory effects of proanthocyanidin-rich red rice extract via suppression of MAPK, AP-1 and NF-κB pathways in Raw 264.7 macrophages
- Aromadendrin Inhibits Lipopolysaccharide-Induced Nuclear Translocation of NF-kappaB and Phosphorylation of JNK in RAW 264.7 Macrophage Cells