Cancer Res Treat.
2018 Jan;50(1):111-117. 10.4143/crt.2017.001.
Proteomic Biomarkers for Bisphenol A–Early Exposure and Women’s Thyroid Cancer
- Affiliations
-
- 1Department of Toxicology, Research Center for Cell Fate Control, College of Pharmacy, Sookmyung Women's University, Seoul, Korea. myang@sm.ac.kr
- 2Department of Otolaryngology, Hanyang University College of Medicine, Seoul, Korea.
- 3Department of Anatomy and Pathology, Research Center for Cell Fate Control, College of Pharmacy, Sookmyung Women's University, Seoul, Korea.
- 4Department of Cancer Epidemiology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA.
- 5Department of Biomedical and Pharmaceutical Sciences, College of Health Professions and Biomedical Sciences, University of Montana, Missoula, MT, USA.
- KMID: 2403480
- DOI: http://doi.org/10.4143/crt.2017.001
Abstract
- PURPOSE
For the target treatment and prevention of women's increased thyroid cancer, we focused on risks of environmental exposure to endocrine disrupting chemicals, particularly bisphenol A (BPA), and its high susceptible exposure-timing, particularly early exposure in lives.
MATERIALS AND METHODS
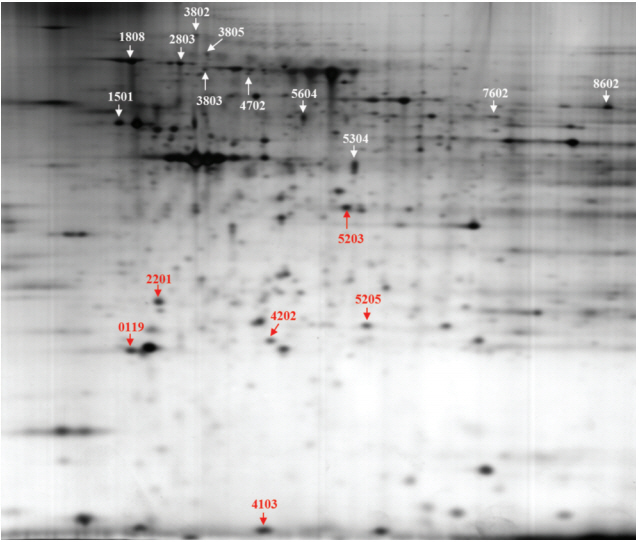
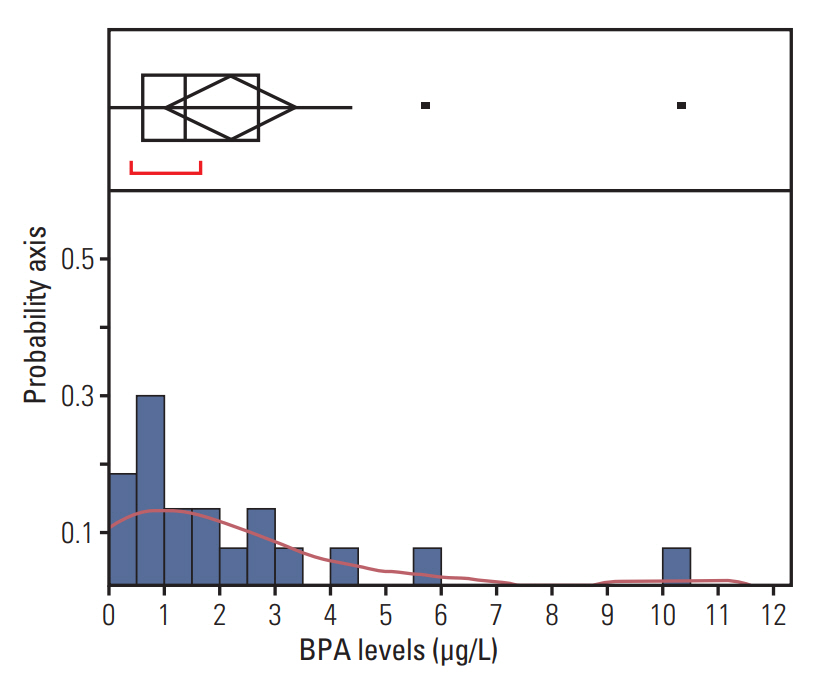
Female ICR mice were exposed to BPA in utero and in early life (15, 75, and 300 mg/L of drinking water via pregnant mice and lactation). We identified BPA-responsive proteins in mice thyroid by two-dimensional gel electrophoresis, image analyses, and electrospray ionization quadrupole time-of-flight mass spectrometry. We further analyzed expression of the BPA-responsive proteins in women thyroid cancer patients (n=28).
RESULTS
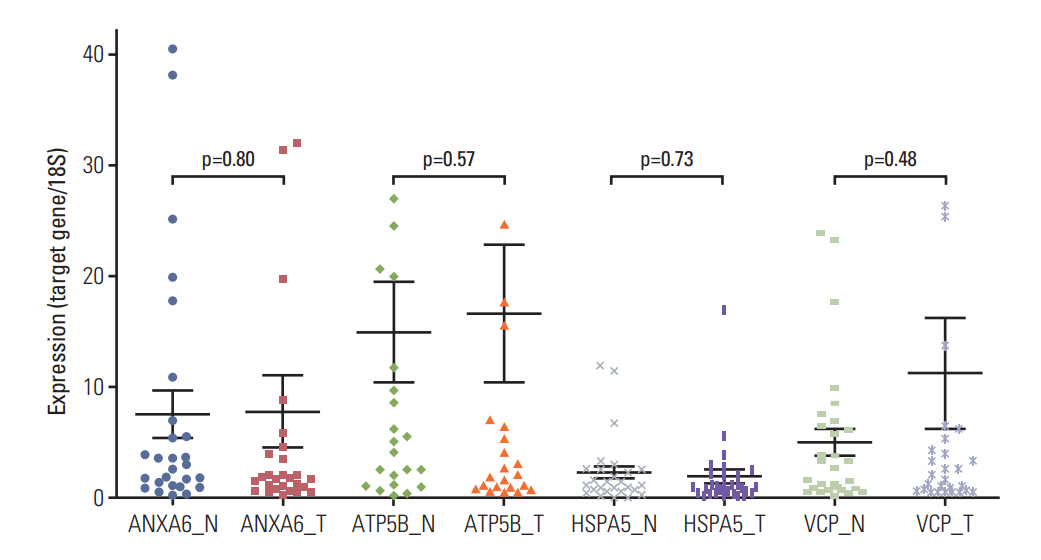
We found the altered 17 proteins in BPA dose-dependent manner among the thyroid tissues of offspring mice and identified nine proteins of them, including Anxa6, Atp5b, Hspa5, and Vcp, etc. In addition, we observed the positive association between blood BPA levels and mRNA expression of the ANXA6 and VCP not in normal but thyroid cancer tissues.
CONCLUSION
Our study provides ANXA6 and VCP as proteomic biomarkers for BPA-early life exposure and their potential for women's thyroid cancer.
Keyword
MeSH Terms
-
Animals
Annexin A6
Biomarkers*
Drinking Water
Electrophoresis, Gel, Two-Dimensional
Endocrine Disruptors
Environmental Exposure
Female
Humans
Mass Spectrometry
Maternal Exposure
Mice
Mice, Inbred ICR
Proteomics
RNA, Messenger
Thyroid Gland*
Thyroid Neoplasms*
Annexin A6
Biomarkers
Drinking Water
Endocrine Disruptors
RNA, Messenger
Figure
Reference
-
References
1. Lee DW, Oh WY, Yi SH, Ku B, Lee MY, Cho YH, et al. Estimation of bisphenol A: human toxicity by 3D cell culture arrays, high throughput alternatives to animal tests. Toxicol Lett. 2016; 259:87–94.2. Yi B, Kim C, Yang M. Biological monitoring of bisphenol A with HLPC/FLD and LC/MS/MS assays. J Chromatogr B Analyt Technol Biomed Life Sci. 2010; 878:2606–10.
Article3. Ghotbaddini M, Powell JB. The AhR ligand, TCDD, regulates androgen receptor activity differently in androgen-sensitive versus castration-resistant human prostate cancer cells. Int J Environ Res Public Health. 2015; 12:7506–18.
Article4. Andrianou XD, Gangler S, Piciu A, Charisiadis P, Zira C, Aristidou K, et al. Human exposures to bisphenol A, bisphenol F and chlorinated bisphenol A derivatives and thyroid function. PLoS One. 2016; 11:e0155237.
Article5. Andra SS, Makris KC. Thyroid disrupting chemicals in plastic additives and thyroid health. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2012; 30:107–51.
Article6. Yu GP, Li JC, Branovan D, McCormick S, Schantz SP. Thyroid cancer incidence and survival in the national cancer institute surveillance, epidemiology, and end results race/ethnicity groups. Thyroid. 2010; 20:465–73.
Article7. Lee S, Lee YY, Yoon HJ, Choi E, Suh M, Park B, et al. Responses to overdiagnosis in thyroid cancer screening among Korean women. Cancer Res Treat. 2016; 48:883–91.
Article8. Bae JM. Overdiagnosis: epidemiologic concepts and estimation. Epidemiol Health. 2015; 37:e2015004.
Article9. Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol. 2013; 2013:965212.
Article10. Caini S, Gibelli B, Palli D, Saieva C, Ruscica M, Gandini S. Menstrual and reproductive history and use of exogenous sex hormones and risk of thyroid cancer among women: a meta-analysis of prospective studies. Cancer Causes Control. 2015; 26:511–8.
Article11. Oh CM, Jung KW, Won YJ, Shin A, Kong HJ, Lee JS. Age-period-cohort analysis of thyroid cancer incidence in Korea. Cancer Res Treat. 2015; 47:362–9.
Article12. Prins GS, Tang WY, Belmonte J, Ho SM. Perinatal exposure to oestradiol and bisphenol A alters the prostate epigenome and increases susceptibility to carcinogenesis. Basic Clin Pharmacol Toxicol. 2008; 102:134–8.
Article13. Yang M, Kim SY, Chang SS, Lee IS, Kawamoto T. Urinary concentrations of bisphenol A in relation to biomarkers of sensitivity and effect and endocrine-related health effects. Environ Mol Mutagen. 2006; 47:571–8.
Article14. Kim M, Bae M, Na H, Yang M. Environmental toxicants-induced epigenetic alterations and their reversers. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2012; 30:323–67.
Article15. Uauy R, Kain J, Corvalan C. How can the Developmental Origins of Health and Disease (DOHaD) hypothesis contribute to improving health in developing countries? Am J Clin Nutr. 2011; 94(6 Suppl):1759S–64S.
Article16. Feinberg AP, Fallin MD. Epigenetics at the crossroads of genes and the environment. JAMA. 2015; 314:1129–30.
Article17. Lee HS, Pyo MY, Yang M. Set, a putative oncogene, as a biomarker for prenatal exposure to bisphenol A. Asian Pac J Cancer Prev. 2012; 13:2711–5.
Article18. Garcia-Arevalo M, Alonso-Magdalena P, Servitja JM, Boronat-Belda T, Merino B, Villar-Pazos S, et al. Maternal exposure to bisphenol-A during pregnancy increases pancreatic beta-cell growth during early life in male mice offspring. Endocrinology. 2016; 157:4158–71.19. Analytical Methods Committee. Using the Grubbs and Cochran tests to identify outliers. Anal Methods. 2015; 7:7948–50.20. Rahman MS, Kwon WS, Yoon SJ, Park YJ, Ryu BY, Pang MG. A novel approach to assessing bisphenol-A hazards using an in vitro model system. BMC Genomics. 2016; 17:577.
Article21. Yang M, Lee HS, Pyo MY. Proteomic biomarkers for prenatal bisphenol A-exposure in mouse immune organs. Environ Mol Mutagen. 2008; 49:368–73.
Article22. Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci U S A. 2007; 104:13056–61.
Article23. Pozo K, Castro-Rivera E, Tan C, Plattner F, Schwach G, Siegl V, et al. The role of Cdk5 in neuroendocrine thyroid cancer. Cancer Cell. 2013; 24:499–511.
Article24. Lomnytska MI, Becker S, Gemoll T, Lundgren C, Habermann J, Olsson A, et al. Impact of genomic stability on protein expression in endometrioid endometrial cancer. Br J Cancer. 2012; 106:1297–305.
Article25. Sakwe AM, Koumangoye R, Guillory B, Ochieng J. Annexin A6 contributes to the invasiveness of breast carcinoma cells by influencing the organization and localization of functional focal adhesions. Exp Cell Res. 2011; 317:823–37.
Article26. Vila de Muga S, Timpson P, Cubells L, Evans R, Hayes TE, Rentero C, et al. Annexin A6 inhibits Ras signalling in breast cancer cells. Oncogene. 2009; 28:363–77.
Article27. Lorenz S, Eszlinger M, Paschke R, Aust G, Weick M, Fuhrer D, et al. Calcium signaling of thyrocytes is modulated by TSH through calcium binding protein expression. Biochim Biophys Acta. 2010; 1803:352–60.
Article28. Xiong Q, Zhan S, Zhang N, Ge W, Wang T. Protein profiling of papillary thyroid carcinoma with and without lymph node metastasis: a proteomic study. Int J Clin Exp Pathol. 2016; 9:3057–69.29. Meyer H, Bug M, Bremer S. Emerging functions of the VCP/p97 AAA-ATPase in the ubiquitin system. Nat Cell Biol. 2012; 14:117–23.
Article30. Chapman E, Maksim N, de la Cruz F, La Clair JJ. Inhibitors of the AAA+ chaperone p97. Molecules. 2015; 20:3027–49.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Associations Between Thyroid Hormone Levels and Urinary Concentrations of Bisphenol A, F, and S in 6-Year-old Children in Korea
- Bisphenols and Thyroid Hormone
- Proteomic profiling of bladder cancer for precision medicine in the clinical setting: A review for the busy urologist
- A Validation Study of a Multiple Reaction Monitoring-Based Proteomic Assay to Diagnose Breast Cancer
- Identification of Biomarkers for Breast Cancer Using Databases