Cancer Res Treat.
2015 Oct;47(4):687-696. 10.4143/crt.2014.225.
Phase I Study of Axitinib in Combination with Cisplatin and Capecitabine in Patients with Previously Untreated Advanced Gastric Cancer
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Hospital and Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea. bangyj@snu.ac.kr
- 2Division of Gastroenterology, National Cancer Center Hospital East, Chiba, Japan.
- 3Department of Medical Oncology, Oita University Faculty of Medicine, Yufu, Japan.
- 4Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- 5Department of Internal Medicine, Research Institute and Hospital, National Cancer Center, Goyang, Korea.
- 6Pfizer Oncology, San Diego, CA, USA.
- 7Pfizer Oncology, Milan, Italy.
- KMID: 2403387
- DOI: http://doi.org/10.4143/crt.2014.225
Abstract
- PURPOSE
This phase I trial evaluated the question of whether the standard starting dose of axitinib could be administered in combination with therapeutic doses of cisplatin/capecitabine in patients with previously untreated advanced gastric cancer, and assessed overall safety, pharmacokinetics, and preliminary antitumor activity of this combination.
MATERIALS AND METHODS
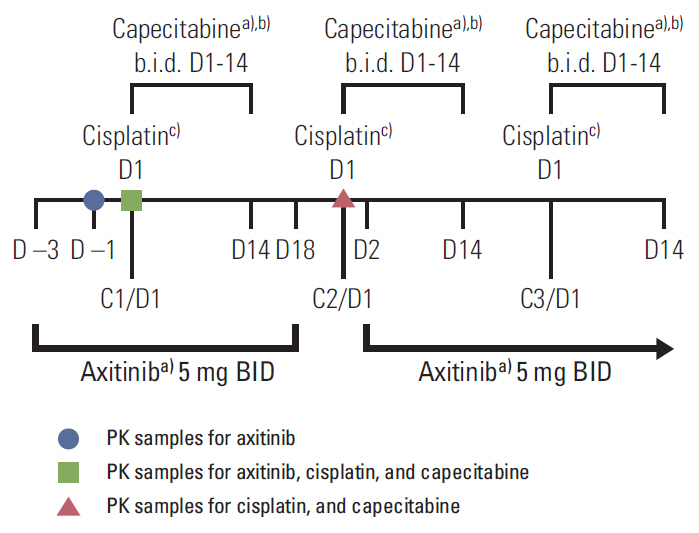
Patients in dose level (DL) 1 received axitinib 5 mg twice a day (days 1 to 21) with cisplatin 80 mg/m2 (day 1) and capecitabine 1,000 mg/m2 twice a day (days 1 to 14) in 21-day cycles. Maximum tolerated dose (MTD) was the highest dose at which < or = 30% of the first 12 patients experienced a dose-limiting toxicity (DLT) during cycle 1. Ten additional patients were enrolled and treated at the MTD in order to obtain additional safety and pharmacokinetic data.
RESULTS
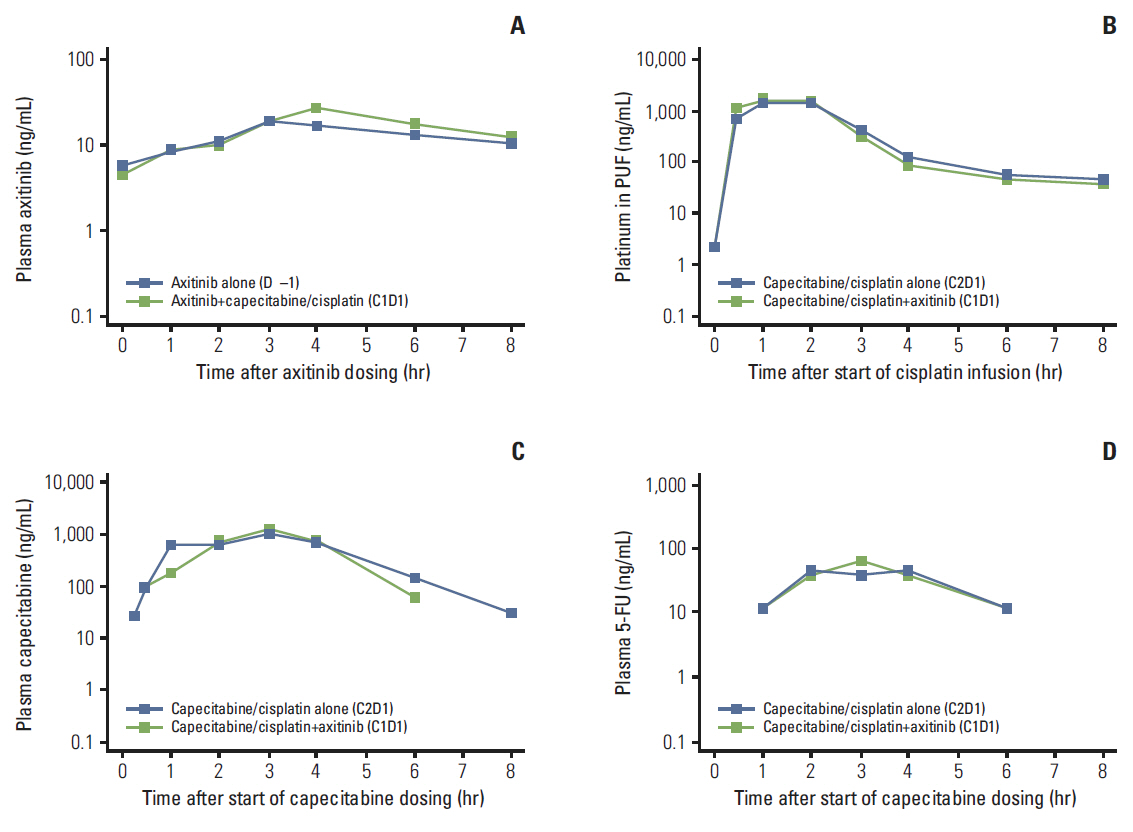
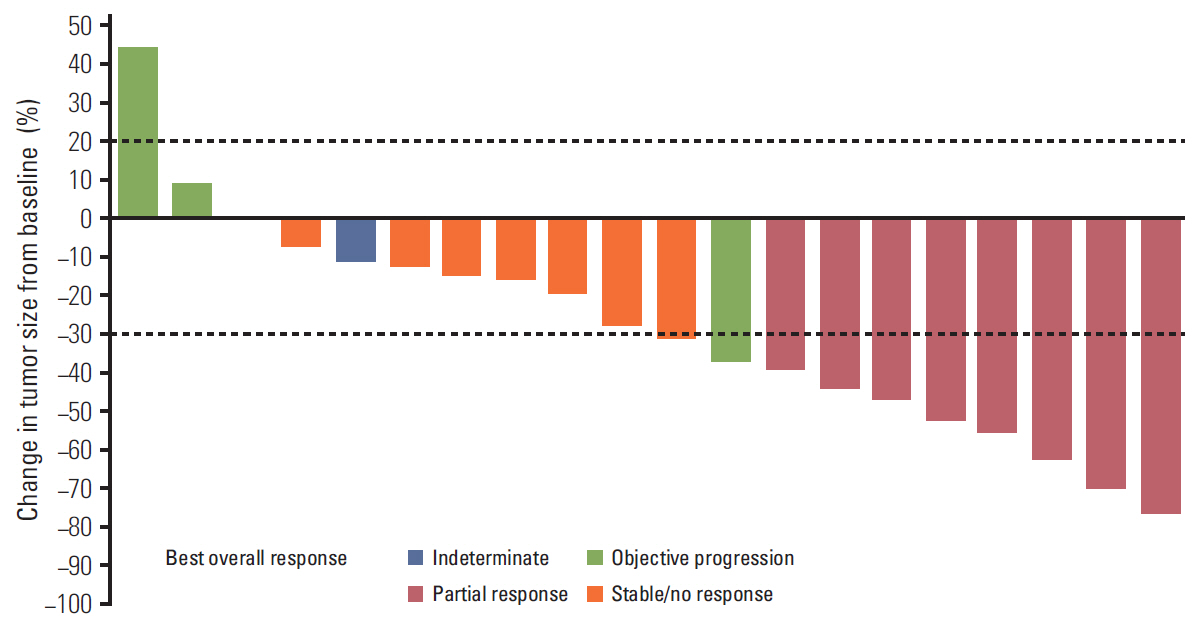
Three DLTs occurred during cycle 1 in three (25%) of the first 12 patients: ruptured abdominal aortic aneurysm, acute renal failure, and > 5 consecutive days of missed axitinib due to thrombocytopenia. DL1 was established as the MTD, since higher DL cohorts were not planned. Common grade 3/4 non-hematologic adverse events in 22 patients treated at DL1 included hypertension (36.4%) and decreased appetite and stomatitis (18.2% each). Cisplatin/capecitabine slightly increased axitinib exposure; axitinib decreased capecitabine and 5-fluorouracil exposure. Eight patients (36.4%) each had partial response or stable disease. Median response duration was 9.1 months; median progression-free survival was 3.8 months.
CONCLUSION
In patients with advanced gastric cancer, standard doses of axitinib plus therapeutic doses of cisplatin and capecitabine could be administered in combination. Adverse events were manageable.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Anti-angiogenic Therapy in Patients with Advanced Gastric and Gastroesophageal Junction Cancer: A Systematic Review
Li-Tzong Chen, Do-Youn Oh, Min-Hee Ryu, Kun-Huei Yeh, Winnie Yeo, Roberto Carlesi, Rebecca Cheng, Jongseok Kim, Mauro Orlando, Yoon-Koo Kang
Cancer Res Treat. 2017;49(4):851-868. doi: 10.4143/crt.2016.176.
Reference
-
References
1. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008 v1.2, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet]. Lyon: International Agency for Research on Cancer;2010. [cited 2014 Mar 6]. Available from: http://globocan.iarc.fr/.2. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN Guidelines™) v2.2013 gastric cancer [Internet]. Fort Washington, PA: National Comprehensive Cancer Network;c2014. [cited 2014 Mar 6]. Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.3. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010; 376:687–97.
Article4. Shi H, Xu JM, Hu NZ, Xie HJ. Prognostic significance of expression of cyclooxygenase-2 and vascular endothelial growth factor in human gastric carcinoma. World J Gastroenterol. 2003; 9:1421–6.
Article5. Maeda K, Chung YS, Ogawa Y, Takatsuka S, Kang SM, Ogawa M, et al. Prognostic value of vascular endothelial growth factor expression in gastric carcinoma. Cancer. 1996; 77:858–63.
Article6. Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011; 29:3968–76.
Article7. Kim C, Lee JL, Choi YH, Kang BW, Ryu MH, Chang HM, et al. Phase I dose-finding study of sorafenib in combination with capecitabine and cisplatin as a first-line treatment in patients with advanced gastric cancer. Invest New Drugs. 2012; 30:306–15.
Article8. Bang YJ, Kang YK, Kang WK, Boku N, Chung HC, Chen JS, et al. Phase II study of sunitinib as second-line treatment for advanced gastric cancer. Invest New Drugs. 2011; 29:1449–58.
Article9. Moehler M, Mueller A, Hartmann JT, Ebert MP, Al-Batran SE, Reimer P, et al. An open-label, multicentre biomarker-oriented AIO phase II trial of sunitinib for patients with chemorefractory advanced gastric cancer. Eur J Cancer. 2011; 47:1511–20.
Article10. Park SR, Lee KW, Oh DY, Park YI, Roh EJ, Khosravan R, et al. Sunitinib (SU) with cisplatin (P) and capecitabine (X) or oxaliplatin (O) and X (XELOX) in advanced gastric cancer (GC): a phase I, dose-finding study. In : the 35th Annual Congress of the European Society for Medical Oncology (ESMO); 2010 Oct 8-12; Milan, Italy. Viganello-Lugano: European Society for Medical Oncology;2010.11. Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014; 383:31–9.
Article12. Hu-Lowe DD, Zou HY, Grazzini ML, Hallin ME, Wickman GR, Amundson K, et al. Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin Cancer Res. 2008; 14:7272–83.
Article13. Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011; 378:1931–9.
Article14. Cohen EE, Rosen LS, Vokes EE, Kies MS, Forastiere AA, Worden FP, et al. Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J Clin Oncol. 2008; 26:4708–13.
Article15. Schiller JH, Larson T, Ou SH, Limentani S, Sandler A, Vokes E, et al. Efficacy and safety of axitinib in patients with advanced non-small-cell lung cancer: results from a phase II study. J Clin Oncol. 2009; 27:3836–41.
Article16. Fruehauf J, Lutzky J, McDermott D, Brown CK, Meric JB, Rosbrook B, et al. Multicenter, phase II study of axitinib, a selective second-generation inhibitor of vascular endothelial growth factor receptors 1, 2, and 3, in patients with metastatic melanoma. Clin Cancer Res. 2011; 17:7462–9.
Article17. Spano JP, Chodkiewicz C, Maurel J, Wong R, Wasan H, Barone C, et al. Efficacy of gemcitabine plus axitinib compared with gemcitabine alone in patients with advanced pancreatic cancer: an open-label randomised phase II study. Lancet. 2008; 371:2101–8.
Article18. Rugo HS, Stopeck AT, Joy AA, Chan S, Verma S, Lluch A, et al. Randomized, placebo-controlled, double-blind, phase II study of axitinib plus docetaxel versus docetaxel plus placebo in patients with metastatic breast cancer. J Clin Oncol. 2011; 29:2459–65.
Article19. Infante JR, Reid TR, Cohn AL, Edenfield WJ, Cescon TP, Hamm JT, et al. Axitinib and/or bevacizumab with modified FOLFOX-6 as first-line therapy for metastatic colorectal cancer: a randomized phase 2 study. Cancer. 2013; 119:2555–63.
Article20. Postel-Vinay S, Gomez-Roca C, Molife LR, Anghan B, Levy A, Judson I, et al. Phase I trials of molecularly targeted agents: should we pay more attention to late toxicities? J Clin Oncol. 2011; 29:1728–35.
Article21. Chen Y, Tortorici MA, Garrett M, Hee B, Klamerus KJ, Pithavala YK. Clinical pharmacology of axitinib. Clin Pharmacokinet. 2013; 52:713–25.
Article22. Vermorken JB, van der Vijgh WJ, Klein I, Hart AA, Gall HE, Pinedo HM. Pharmacokinetics of free and total platinum species after short-term infusion of cisplatin. Cancer Treat Rep. 1984; 68:505–13.23. Go RS, Adjei AA. Review of the comparative pharmacology and clinical activity of cisplatin and carboplatin. J Clin Oncol. 1999; 17:409–22.
Article24. Walko CM, Lindley C. Capecitabine: a review. Clin Ther. 2005; 27:23–44.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chemotherapy of Advanced Gastric Cancer
- Combination chemotherapy with docetaxel and cisplatin as first-line treatment in advanced gastric cancer: is it a new effective chemotherapy?
- Chemotherapy for Advanced Gastric Cancer: Slow but Further Progress
- A Multicenter Randomized Phase II Study of Docetaxel vs. Docetaxel Plus Cisplatin vs. Docetaxel Plus S-1 as Second-Line Chemotherapy in Metastatic Gastric Cancer Patients Who Had Progressed after Cisplatin Plus Either S-1 or Capecitabine
- A New Option for Advanced Gastric Cancer: Docetaxel and Novel Oral Fluoropyrimidine Combination Chemotherapy