J Korean Med Assoc.
2007 Jun;50(6):556-560. 10.5124/jkma.2007.50.6.556.
A New Option for Advanced Gastric Cancer: Docetaxel and Novel Oral Fluoropyrimidine Combination Chemotherapy
- Affiliations
-
- 1Department of Internal Medicine, Hallym University College of Medicine, Korea. fhdzang@hallym.or.kr
- KMID: 2184865
- DOI: http://doi.org/10.5124/jkma.2007.50.6.556
Abstract
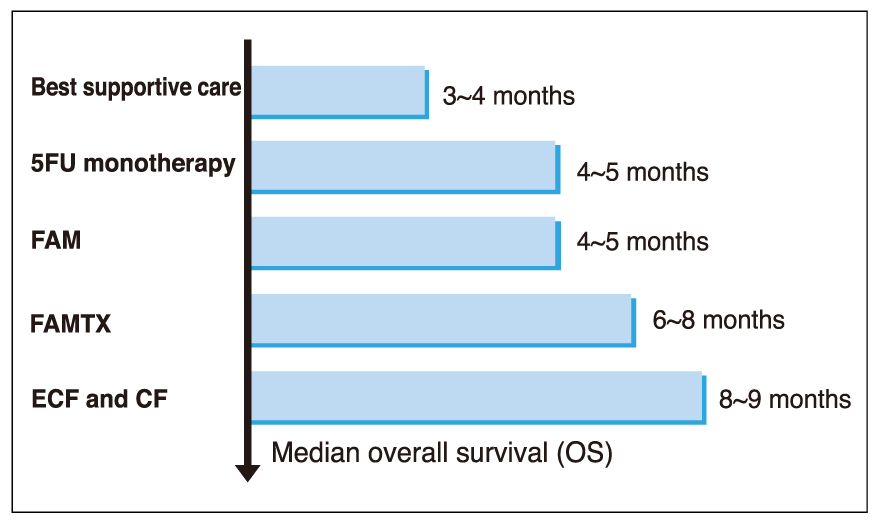
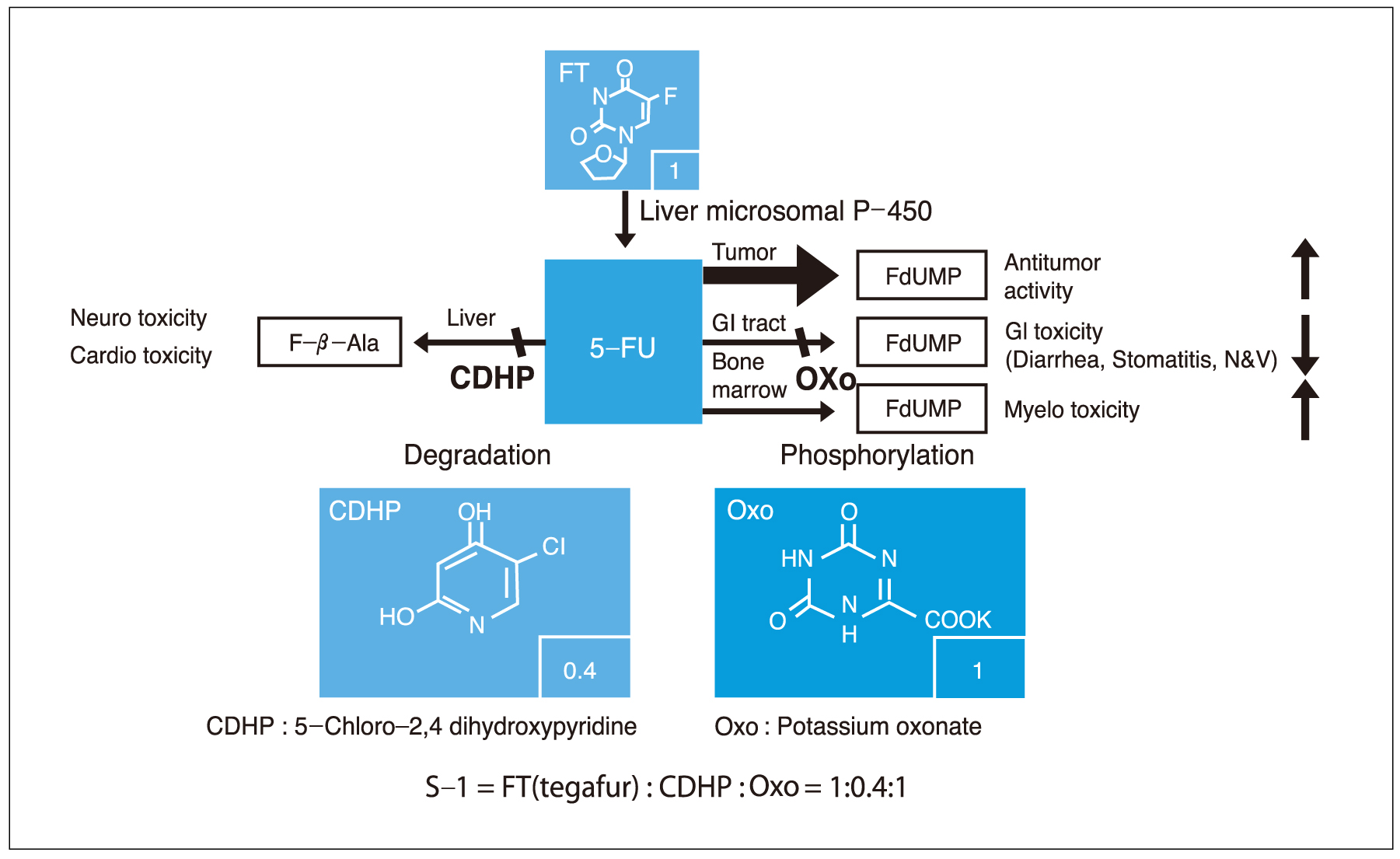
- Although gastric cancer is the most common cancer and the second leading cause of cancer deaths in Korea, the prognosis for advanced gastric cancer remains poor, and there is no established standard front-line chemotherapy for advanced stage. However, many clinical trials have been developed to improve the response rate and survival in patients with advanced gastric cancer. Novel oral fluoropyrimidines, capecitabine and S-1, are substituting inconvenient 5-FU continuous infusions. These oral fluoropyrimidines combined with docetaxel (1-hour infusion) lead to improve anticancer efficacy and convenience. Capecitabine and docetaxel combination regimens showed response rates 39~60% with median survival 9.5~12 months, and major adverse reactions were hand-foot syndrome and neutropenia. Also, S-1 and docetaxel combination regimens showed response rates 46~56% with median survival 14~14.9 months, and major adverse reaction was neutropenia. The combination of docetaxel and novel oral fluoropyrimidine is highly active and well tolerated in patients with advanced gastric cancer. Large randomized clinical trials are warranted to confirm the efficacy of those regimens. Also, we are looking forward to having the results from studies of new chemotherapeutic agents and modalities.
MeSH Terms
Figure
Reference
-
1. Park YH, Ryoo B, Choi S, Kim H. A phase II study of capecitabine and docetaxel combination chemotherapy in patients with advanced gastric cancer. Br J Cancer. 2004. 90:1329–1333.
Article2. Giordano KF, Jatoi A, Stella PJ, Foster N, Tschetter LK, Alberts SR, Dakhil SR, Mailliard JA, Flynn PJ, Nikcevich DA. Docetaxel and capecitabine in patients with metastatic adenocarcinoma of the stomach and gastroesophageal junction: a phase II study from the North Central Cancer Treatment Group. Ann Oncol. 2006. 17:652–656.
Article3. Chun JH, Kim HK, Lee JS, Choi JY, Hwangbo B, Lee HG, Park SR, Choi IJ, Kim CG, Ryu KW, Kim Y, Lee JS, Bae J. Weekly docetaxel in combination with capecitabine in patients with metastatic gastric cancer. Am J Clin Oncol. 2005. 28:188–194.
Article4. Yamaguchi K, Shimamura T, Hydo I, Koizumi W, Doi T, Narahara H, Komatsu Y, Kato T, Saitoh S, Akiya T, Munakata M, Miyata Y, Maeda Y, Takiuchi H, Nakano S, Esaki T, Kinjo F, Sakata Y. Phase I/II study of docetaxel and S-1 in patients with advanced gastric cancer. Br J Cancer. 2006. 94:1803–1808.
Article5. Yoshida K, Ninomiya M, Takakura N, Hirabayashi N, Takiyama W, Sato Y, Todo S, Terashima M, Gotoh M, Sakamoto J, Nishiyama M. Phase II study of docetaxel and S-1 combination therapy for advanced or recurrent gastric cancer. Clin Cancer Res. 2006. 12:3402–3407.
Article6. Zang D, Song S, Kwon J, Jung J, Kim H, Kim J, Shin H, Park Y. Phase I/II trial with docetaxel and S-1 for patients with advanced or recurrent gastric cancer. Ann Oncol. 2006. 17:S. ix314.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Combination chemotherapy with docetaxel and cisplatin as first-line treatment in advanced gastric cancer: is it a new effective chemotherapy?
- Chemotherapy for Advanced Gastric Cancer in Elderly Patients
- Curative Resection of Inoperable, Locally Advanced Gastric Cancer after Neoadjuvant Chemotherapy with Taxotere and Cisplatin
- Treatment for unresectable gastric cancer
- Recent Advances in Chemotherapy of Gastric Cancer