Int J Stem Cells.
2017 Nov;10(2):160-168. 10.15283/ijsc17014.
Treatment with Allogenic Mesenchymal Stromal Cells in a Murine Model of Systemic Lupus Erythematosus
- Affiliations
-
- 1Rheumatology Unit, Department of Clinical and Experimental Medicine, University of Pisa, Pisa, Italy. chiara.tani@for.unipi.it
- 2Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, iPATH. Berlin, core unit of the Charité. Berlin, Germany.
- 3Haematology Unit, Department of Clinical and Experimental Medicine, University of Pisa, Pisa, Italy.
- KMID: 2400870
- DOI: http://doi.org/10.15283/ijsc17014
Abstract
OBJECTIVE
Pre-clinical and uncontrolled studies in patients with systemic lupus erythematosus (SLE) showed that mesenchymal stromal cells (MSCs) have a potential therapeutic role in refractory cases. The optimal therapeutic strategy in these patients remain to be elucidated. Our aim was to test the hypothesis that repeated administrations of 1×10â¶/kg body weight of allogenic MSCs, that is a significantly lower dosage with respect to the fixed 1×10ⶠMSC used in animal models, can be effective in improving the clinical course of a murine SLE model.
METHODS
Bone marrow derived MSCs were obtained from 12-week-old C57BL/6J mice. Seventy-five 8 weeks old female NZ mice were randomly assigned to receive via caudal vein the following alternative treatments: 1) single infusion of 10ⶠMSCs/kg body weight at 18 weeks of age (NZ(s18)) or at at 22 weeks of age (NZ(s22)); 2) multiple monthly infusions of 10ⶠMSCs/kg body weight starting at 18 weeks of age (NZ(M18)) or at 22 weeks of age (NZ(M22)); 3) saline infusions (NZ(c)) Fifteen 8 weeks old C57BL/6J mice (Envigo, Huntingdon, UK) were used as untreated controls (C). Weekly, body weight was recorded and twenty-four hour urines were collected by metabolic cages for each animal; proteinuria was detected by dipstick analysis. At sacrifice, peripheral blood samples were collected from mice and anti-dsDNA antibodies were detected by enzyme immunoassorbent assay (ELISA) method using commercial kits. At sacrifice, kidneys were analyzed for histopathology and immunohistochemical analysis for B220, CD4, MPO, CD4âºFoxp3, F40/80 infiltration was performed.
RESULTS
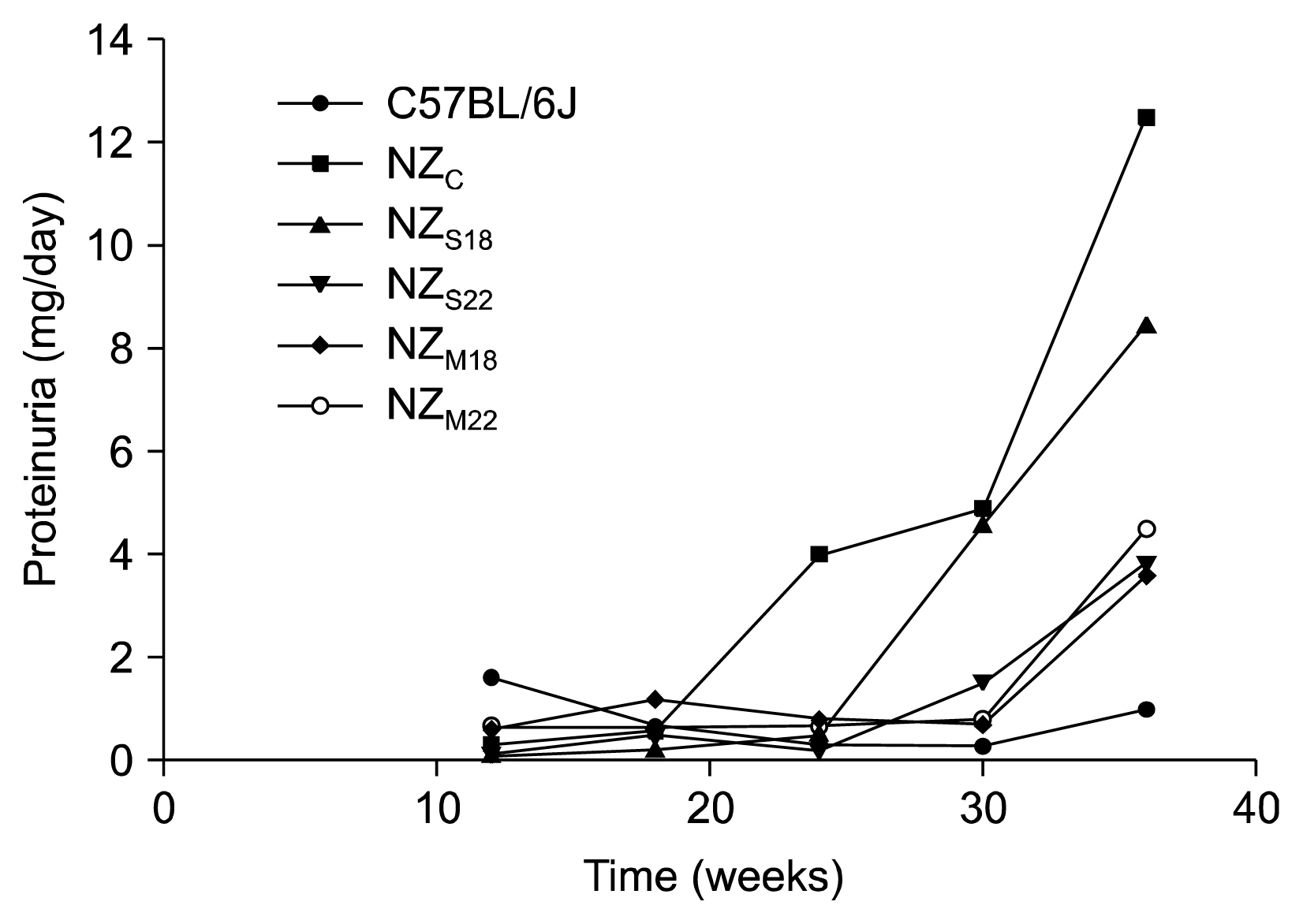
Proteinuria occurrence was delayed NZ(S) and NZ(M) mice, no differences were observed in anti-dsDNA autoantibody titer among the groups at the different time-points; at 36 weeks, no significant differences were observed in term of nephritis scores. Inflammatory cells deposition (MPO and F4/80 positive cells) in NZM was significantly higher than in NZ and NZ(S). An overexpression of B lymphocytes (B220) was found in NZ(M) while T regulatory cells (CD4⺠Foxp3⺠cells) were reduced in both NZ(S) and NZ(M) with respect to NZ(c).
CONCLUSIONS
Overall, our study failed to show a positive effect of a treatment with murine MSCs in this model and, for some aspects, even deleterious results seem to be observed.
MeSH Terms
Figure
Reference
-
References
1. Lisnevskaia L, Murphy G, Isenberg D. Systemic lupus erythematosus. Lancet. 2014; 384:1878–1888. DOI: 10.1016/S0140-6736(14)60128-8. PMID: 24881804.
Article2. Alchi B, Jayne D, Labopin M, Demin A, Sergeevicheva V, Alexander T, Gualandi F, Gruhn B, Ouyang J, Rzepecki P, Held G, Sampol A, Voswinkel J, Ljungman P, Fassas A, Badoglio M, Saccardi R, Farge D. EBMT Autoimmune Disease Working Party Members. Autologous haematopoietic stem cell transplantation for systemic lupus erythematosus: data from the European Group for Blood and Marrow Transplantation registry. Lupus. 2013; 22:245–253. DOI: 10.1177/0961203312470729.
Article3. Gu F, Wang D, Zhang H, Feng X, Gilkeson GS, Shi S, Sun L. Allogeneic mesenchymal stem cell transplantation for lupus nephritis patients refractory to conventional therapy. Clin Rheumatol. 2014; 33:1611–1619. DOI: 10.1007/s10067-014-2754-4. PMID: 25119864.
Article4. Li X, Wang D, Liang J, Zhang H, Sun L. Mesenchymal SCT ameliorates refractory cytopenia in patients with systemic lupus erythematosus. Bone Marrow Transplant. 2013; 48:544–550. DOI: 10.1038/bmt.2012.184.
Article5. Wang D, Niu L, Feng X, Yuan X, Zhao S, Zhang H, Liang J, Zhao C, Wang H, Hua B, Sun L. Long-term safety of umbilical cord mesenchymal stem cells transplantation for systemic lupus erythematosus: a 6-year follow-up study. Clin Exp Med. 2016; [Epub ahead of print].
Article6. Le Blanc K, Ringdén O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007; 262:509–525. DOI: 10.1111/j.1365-2796.2007.01844.x. PMID: 17949362.
Article7. Moll G, Rasmusson-Duprez I, von Bahr L, Connolly-Andersen AM, Elgue G, Funke L, Hamad OA, Lönnies H, Magnusson PU, Sanchez J, Teramura Y, Nilsson-Ekdahl K, Ringdén O, Korsgren O, Nilsson B, Le Blanc K. Are therapeutic human mesenchymal stromal cells compatible with human blood? Stem Cells. 2012; 30:1565–1574. DOI: 10.1002/stem.1111. PMID: 22522999.
Article8. Moll G, Alm JJ, Davies LC, von Bahr L, Heldring N, Stenbeck-Funke L, Hamad OA, Hinsch R, Ignatowicz L, Locke M, Lönnies H, Lambris JD, Teramura Y, Nilsson-Ekdahl K, Nilsson B, Le Blanc K. Do cryopreserved mesenchymal stromal cells display impaired immunomodulatory and therapeutic properties? Stem Cells. 2014; 32:2430–2442. DOI: 10.1002/stem.1729. PMID: 24805247. PMCID: 4381870.
Article9. Prockop DJ, Brenner M, Fibbe WE, Horwitz E, Le Blanc K, Phinney DG, Simmons PJ, Sensebe L, Keating A. Defining the risks of mesenchymal stromal cell therapy. Cytotherapy. 2010; 12:576–578. DOI: 10.3109/14653249.2010.507330. PMID: 20735162.
Article10. von Bahr L, Sundberg B, Lönnies L, Sander B, Karbach H, Hägglund H, Ljungman P, Gustafsson B, Karlsson H, Le Blanc K, Ringdén O. Long-term complications, immunologic effects, and role of passage for outcome in mesenchymal stromal cell therapy. Biol Blood Marrow Transplant. 2012; 18:557–564. DOI: 10.1016/j.bbmt.2011.07.023.
Article11. Lundberg J, Södersten E, Sundström E, Le Blanc K, Andersson T, Hermanson O, Holmin S. Targeted intra-arterial transplantation of stem cells to the injured CNS is more effective than intravenous administration: engraftment is dependent on cell type and adhesion molecule expression. Cell Transplant. 2012; 21:333–343. DOI: 10.3727/096368911X576036.
Article12. Collins E, Gu F, Qi M, Molano I, Ruiz P, Sun L, Gilkeson GS. Differential efficacy of human mesenchymal stem cells based on source of origin. J Immunol. 2014; 193:4381–4390. DOI: 10.4049/jimmunol.1401636. PMID: 25274529. PMCID: 4201962.
Article13. Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012; 21:2724–2752. DOI: 10.1089/scd.2011.0722. PMID: 22468918.
Article14. Woodworth TG, Furst DE. Safety and feasibility of umbilical cord mesenchymal stem cells in treatment-refractory systemic lupus erythematosus nephritis: time for a double-blind placebo-controlled trial to determine efficacy. Arthritis Res Ther. 2014; 16:113. DOI: 10.1186/ar4677. PMID: 25166210. PMCID: 4261567.
Article15. Soleimani M, Nadri S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc. 2009; 4:102–106. DOI: 10.1038/nprot.2008.221. PMID: 19131962.
Article16. Tropel P, Noël D, Platet N, Legrand P, Benabid AL, Berger F. Isolation and characterisation of mesenchymal stem cells from adult mouse bone marrow. Exp Cell Res. 2004; 295:395–406. DOI: 10.1016/j.yexcr.2003.12.030. PMID: 15093739.
Article17. Tao X, Fan F, Hoffmann V, Longo NS, Lipsky PE. Therapeutic impact of the ethyl acetate extract of Tripterygium wilfordii Hook F on nephritis in NZB/W F1 mice. Arthritis Res Ther. 2006; 8:R24. DOI: 10.1186/ar1879. PMID: 16507125. PMCID: 1526566.
Article18. Chang JW, Hung SP, Wu HH, Wu WM, Yang AH, Tsai HL, Yang LY, Lee OK. Therapeutic effects of umbilical cord blood-derived mesenchymal stem cell transplantation in experimental lupus nephritis. Cell Transplant. 2011; 20:245–257. DOI: 10.3727/096368910X520056.
Article19. Choi EW, Shin IS, Park SY, Park JH, Kim JS, Yoon EJ, Kang SK, Ra JC, Hong SH. Reversal of serologic, immunologic, and histologic dysfunction in mice with systemic lupus erythematosus by long-term serial adipose tissue-derived mesenchymal stem cell transplantation. Arthritis Rheum. 2012; 64:243–253. DOI: 10.1002/art.33313.
Article20. Gu Z, Akiyama K, Ma X, Zhang H, Feng X, Yao G, Hou Y, Lu L, Gilkeson GS, Silver RM, Zeng X, Shi S, Sun L. Transplantation of umbilical cord mesenchymal stem cells alleviates lupus nephritis in MRL/lpr mice. Lupus. 2010; 19:1502–1514. DOI: 10.1177/0961203310373782. PMID: 20647254.
Article21. Gu F, Molano I, Ruiz P, Sun L, Gilkeson GS. Differential effect of allogeneic versus syngeneic mesenchymal stem cell transplantation in MRL/lpr and (NZB/NZW)F1 mice. Clin Immunol. 2012; 145:142–152. DOI: 10.1016/j.clim.2012.08.012. PMID: 23041504.
Article22. Jang E, Jeong M, Kim S, Jang K, Kang BK, Lee DY, Bae SC, Kim KS, Youn J. Infusion of human bone marrow-derived mesenchymal stem cells alleviates autoimmune nephritis in a lupus model by suppressing follicular helper T-cell development. Cell Transplant. 2016; 25:1–15. DOI: 10.3727/096368915X688173.
Article23. Ji S, Guo Q, Han Y, Tan G, Luo Y, Zeng F. Mesenchymal stem cell transplantation inhibits abnormal activation of Akt/GSK3β signaling pathway in T cells from systemic lupus erythematosus mice. Cell Physiol Biochem. 2012; 29:705–712. DOI: 10.1159/000178590.
Article24. Ma X, Che N, Gu Z, Huang J, Wang D, Liang J, Hou Y, Gilkeson G, Lu L, Sun L. Allogenic mesenchymal stem cell transplantation ameliorates nephritis in lupus mice via inhibition of B-cell activation. Cell Transplant. 2013; 22:2279–2290. DOI: 10.3727/096368912X658692.
Article25. Youd M, Blickarz C, Woodworth L, Touzjian T, Edling A, Tedstone J, Ruzek M, Tubo R, Kaplan J, Lodie T. Allogeneic mesenchymal stem cells do not protect NZBxNZW F1 mice from developing lupus disease. Clin Exp Immunol. 2010; 161:176–186. PMID: 20456409. PMCID: 2940163.
Article26. Zhou K, Zhang H, Jin O, Feng X, Yao G, Hou Y, Sun L. Transplantation of human bone marrow mesenchymal stem cell ameliorates the autoimmune pathogenesis in MRL/lpr mice. Cell Mol Immunol. 2008; 5:417–424. DOI: 10.1038/cmi.2008.52.
Article27. Schena F, Gambini C, Gregorio A, Mosconi M, Reverberi D, Gattorno M, Casazza S, Uccelli A, Moretta L, Martini A, Traggiai E. Interferon-γ-dependent inhibition of B cell activation by bone marrow-derived mesenchymal stem cells in a murine model of systemic lupus erythematosus. Arthritis Rheum. 2010; 62:2776–2786. DOI: 10.1002/art.27560. PMID: 20496367.
Article28. Che N, Li X, Zhang L, Liu R, Chen H, Gao X, Shi S, Chen W, Sun L. Impaired B cell inhibition by lupus bone marrow mesenchymal stem cells is caused by reduced CCL2 expression. J Immunol. 2014; 193:5306–5314. DOI: 10.4049/jimmunol.1400036. PMID: 25339674.
Article29. Li X, Liu L, Meng D, Wang D, Zhang J, Shi D, Liu H, Xu H, Lu L, Sun L. Enhanced apoptosis and senescence of bone-marrow-derived mesenchymal stem cells in patients with systemic lupus erythematosus. Stem Cells Dev. 2012; 21:2387–2394. DOI: 10.1089/scd.2011.0447. PMID: 22375903.
Article30. Nie Y, Lau C, Lie A, Chan G, Mok M. Defective phenotype of mesenchymal stem cells in patients with systemic lupus erythematosus. Lupus. 2010; 19:850–859. DOI: 10.1177/0961203310361482. PMID: 20511276.
Article31. Geng L, Li X, Feng X, Zhang J, Wang D, Chen J, Liu R, Chen H, Sun L. Association of TNF-α with impaired migration capacity of mesenchymal stem cells in patients with systemic lupus erythematosus. J Immunol Res. 2014; 2014:169082. DOI: 10.1155/2014/169082.32. Wang D, Li J, Zhang Y, Zhang M, Chen J, Li X, Hu X, Jiang S, Shi S, Sun L. Umbilical cord mesenchymal stem cell transplantation in active and refractory systemic lupus erythematosus: a multicenter clinical study. Arthritis Res Ther. 2014; 16:R79. DOI: 10.1186/ar4520. PMID: 24661633. PMCID: 4060570.
Article33. Pacini S. Deterministic and stochastic approaches in the clinical application of mesenchymal stromal cells (MSCs). Front Cell Dev Biol. 2014; 2:50. DOI: 10.3389/fcell.2014.00050. PMID: 25364757. PMCID: 4206995.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Transverse Myelitis as a First Manifestation of Systemic Lupus Erythematosus
- A Case of Lupus Enteritis That Developed during the Treatment of Systemic Lupus Erythematosus
- A Case Of Systemic Lupus Erythematosus Associated With Hyperthyroidism And Severe Retinopathy
- Multiple Dermatofibromas in a woman with Systemic Lupus Erythematosus
- Multiple Dermatofibromas in a Patient with Systemic Lupus Erythematosus