Immune Netw.
2017 Oct;17(5):326-342. 10.4110/in.2017.17.5.326.
Distinct Effects of Monophosphoryl Lipid A, Oligodeoxynucleotide CpG, and Combination Adjuvants on Modulating Innate and Adaptive Immune Responses to Influenza Vaccination
- Affiliations
-
- 1Vaccine Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892, USA.
- 2Center for Inflammation, Immunity & Infection, Institute for Biomedical Sciences, Georgia State University, Atlanta, GA 30303, USA. skang24@gsu.edu
- KMID: 2400638
- DOI: http://doi.org/10.4110/in.2017.17.5.326
Abstract
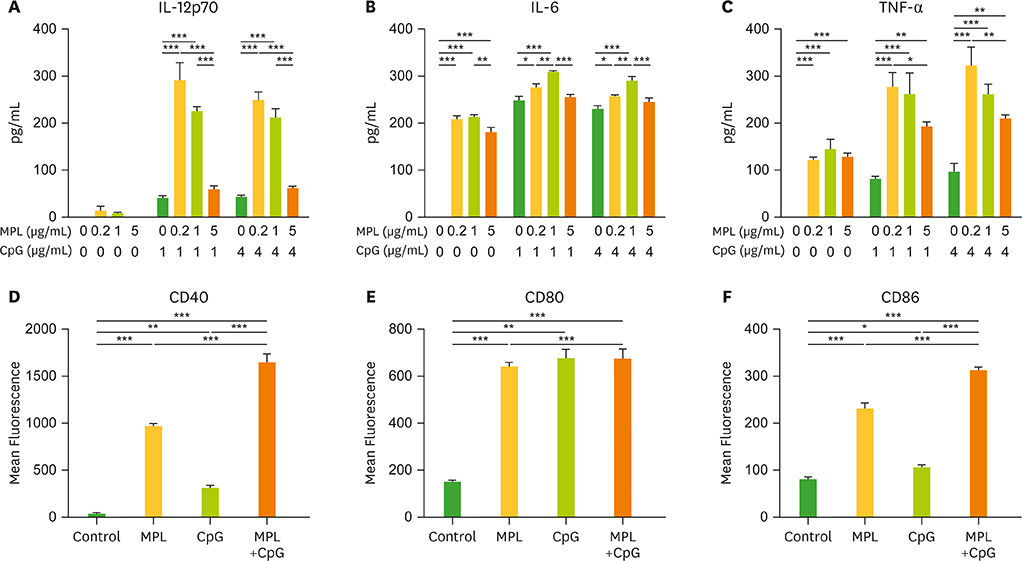
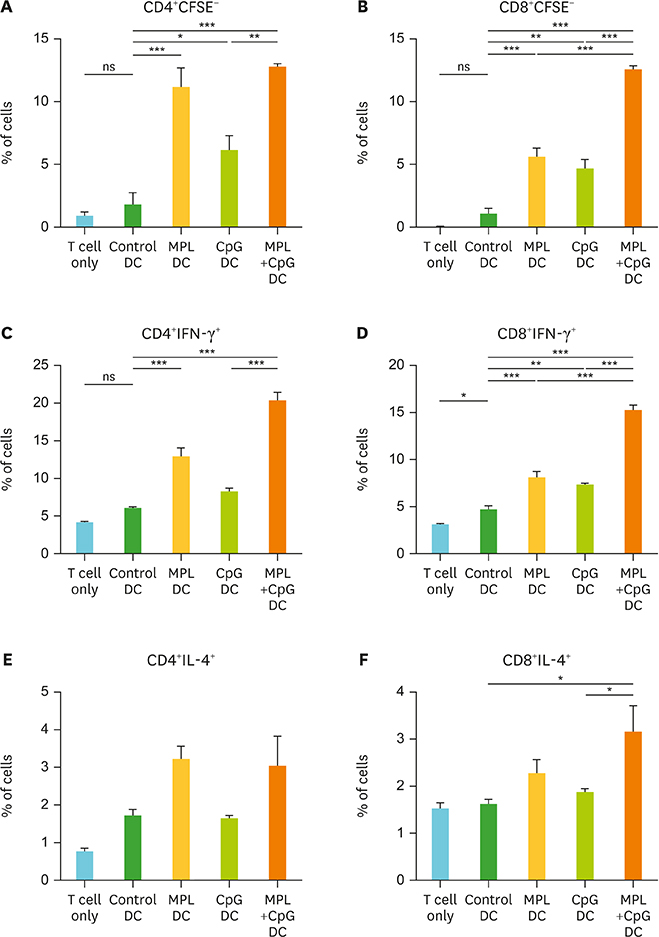
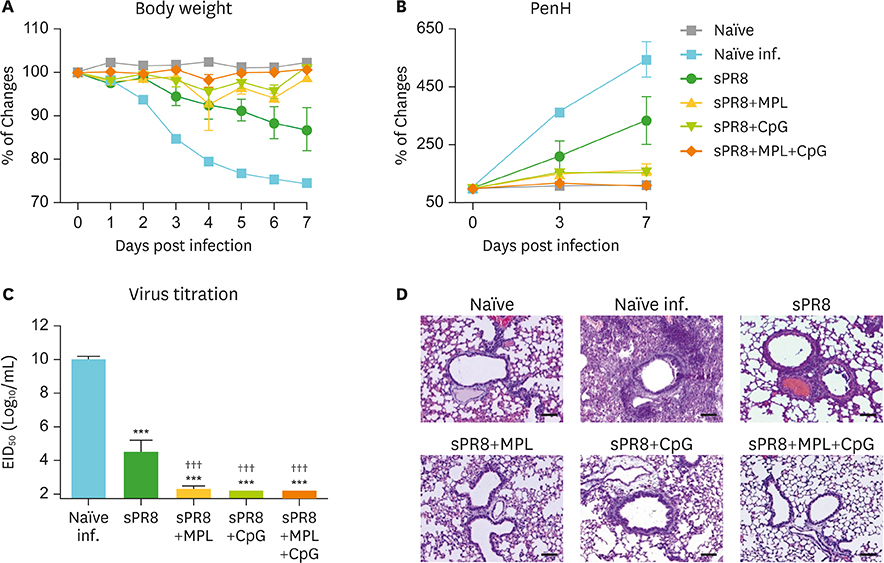
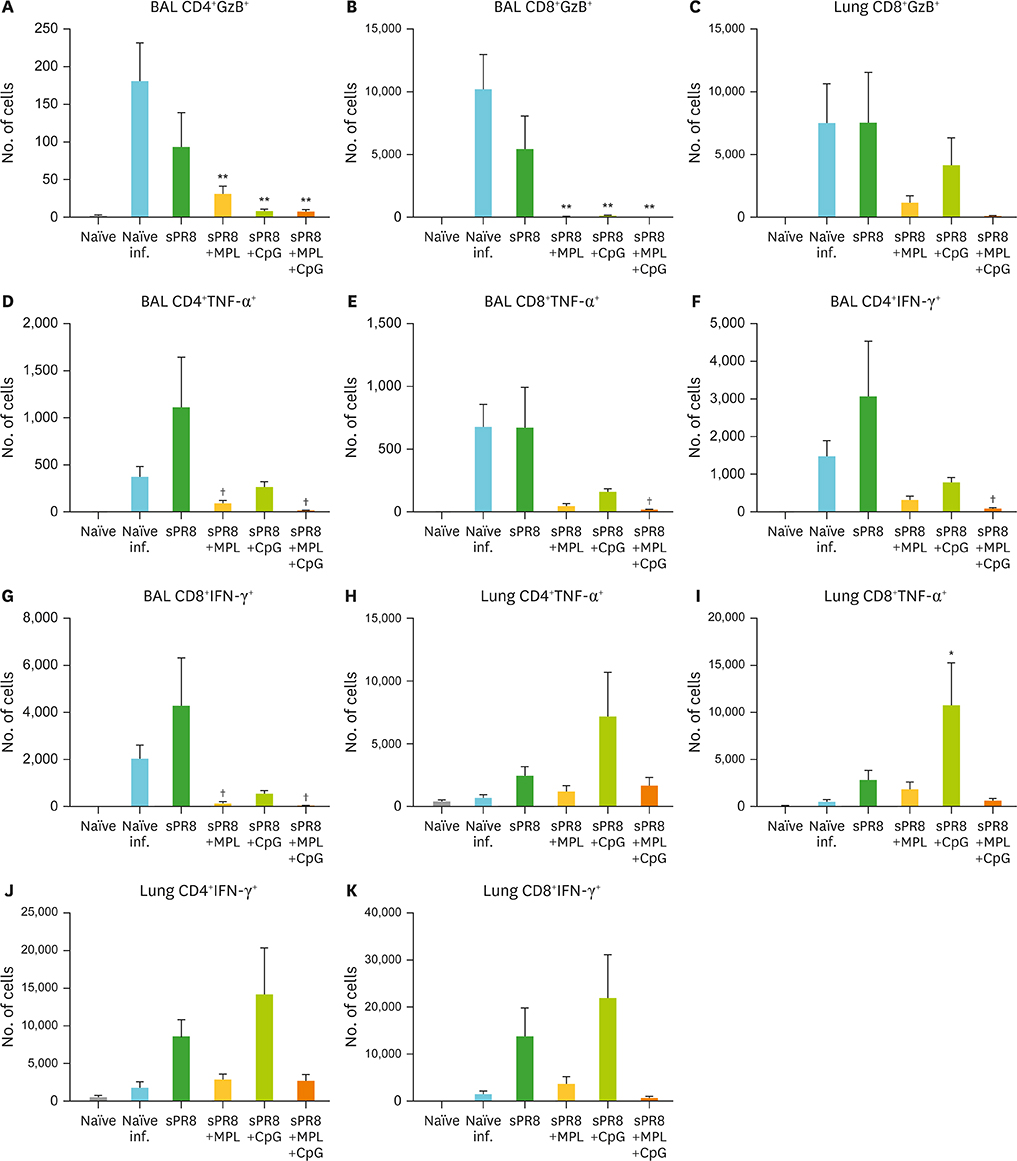
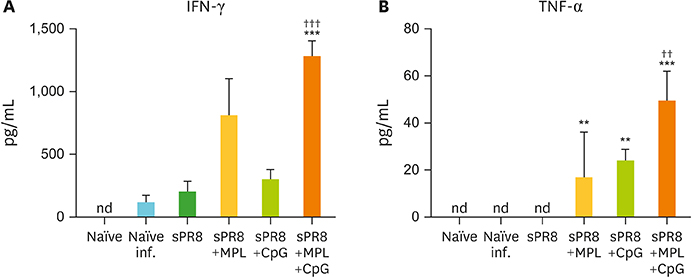
- Monophosphoryl lipid A (MPL) and oligodeoxynucleotide CpG are toll-like receptor (TLR) 4 and 9 agonist, respectively. Here, we investigated the effects of MPL, CpG, and combination adjuvants on stimulating in vitro dendritic cells (DCs), in vivo innate and adaptive immune responses, and protective efficacy of influenza vaccination. Combination of MPL and CpG was found to exhibit distinct effects on stimulating DCs in vitro to secrete IL-12p70 and tumor necrosis factor (TNF)-α and proliferate allogeneic CD8 T cells. Prime immunization of mice with inactivated split influenza vaccine in the presence of low dose MPL+CpG adjuvants increased the induction of virus-specific IgG and IgG2a isotype antibodies. MPL and CpG adjuvants contribute to improving the efficacy of prime influenza vaccination against lethal influenza challenge as determined by body weight monitoring, lung function, viral titers, and histology. A combination of MPL and CpG adjuvants was effective in improving vaccine efficacy as well as in reducing inflammatory immune responses locally and in inducing cellular immune responses upon lethal influenza virus challenge. This study demonstrates unique adjuvant effects of MPL, CpG, and combination adjuvants on modulating innate and adaptive immune responses to influenza prime vaccination.
MeSH Terms
-
Animals
Antibodies
Body Weight
Dendritic Cells
Immunity, Cellular
Immunization
Immunoglobulin G
In Vitro Techniques
Influenza Vaccines
Influenza, Human*
Lipid A*
Lung
Mice
Orthomyxoviridae
T-Lymphocytes
Toll-Like Receptors
Tumor Necrosis Factor-alpha
Vaccination*
Antibodies
Immunoglobulin G
Influenza Vaccines
Lipid A
Toll-Like Receptors
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Centers for Disease Control and Prevention (CDC). Estimates of deaths associated with seasonal influenza --- United States, 1976–2007. MMWR Morb Mortal Wkly Rep. 2010; 59:1057–1062.2. Domínguez A, Godoy P, Torner N. The effectiveness of influenza vaccination in different groups. Expert Rev Vaccines. 2016; 15:751–764.
Article3. Heikkinen T, Heinonen S. Effectiveness and safety of influenza vaccination in children: European perspective. Vaccine. 2011; 29:7529–7534.
Article4. Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010; 11:373–384.
Article5. Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010; 33:492–503.
Article6. Heer AK, Shamshiev A, Donda A, Uematsu S, Akira S, Kopf M, Marsland BJ. TLR signaling fine-tunes anti-influenza B cell responses without regulating effector T cell responses. J Immunol. 2007; 178:2182–2191.
Article7. Steinhagen F, Kinjo T, Bode C, Klinman DM. TLR-based immune adjuvants. Vaccine. 2011; 29:3341–3355.
Article8. Querec T, Bennouna S, Alkan S, Laouar Y, Gorden K, Flavell R, Akira S, Ahmed R, Pulendran B. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J Exp Med. 2006; 203:413–424.
Article9. Godaly G, Young DB. Mycobacterium bovis bacille Calmette Guerin infection of human neutrophils induces CXCL8 secretion by MyD88-dependent TLR2 and TLR4 activation. Cell Microbiol. 2005; 7:591–601.
Article10. Kundi M. New hepatitis B vaccine formulated with an improved adjuvant system. Expert Rev Vaccines. 2007; 6:133–140.
Article11. Szarewski A. Cervarix®: a bivalent vaccine against HPV types 16 and 18, with cross-protection against other high-risk HPV types. Expert Rev Vaccines. 2012; 11:645–657.
Article12. McAleer JP, Vella AT. Educating CD4 T cells with vaccine adjuvants: lessons from lipopolysaccharide. Trends Immunol. 2010; 31:429–435.
Article13. Chu RS, Targoni OS, Krieg AM, Lehmann PV, Harding CV. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J Exp Med. 1997; 186:1623–1631.
Article14. Heeg K, Zimmermann S. CpG DNA as a Th1 trigger. Int Arch Allergy Immunol. 2000; 121:87–97.
Article15. Ioannou XP, Gomis SM, Karvonen B, Hecker R, Babiuk LA, van Drunen Littel-van den Hurk S. CpG-containing oligodeoxynucleotides, in combination with conventional adjuvants, enhance the magnitude and change the bias of the immune responses to a herpesvirus glycoprotein. Vaccine. 2002; 21:127–137.
Article16. Vollmer J, Krieg AM. Immunotherapeutic applications of CpG oligodeoxynucleotide TLR9 agonists. Adv Drug Deliv Rev. 2009; 61:195–204.
Article17. Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Epidemiol. 1938; 27:493–497.18. Quan FS, Yoo DG, Song JM, Clements JD, Compans RW, Kang SM. Kinetics of immune responses to influenza virus-like particles and dose-dependence of protection with a single vaccination. J Virol. 2009; 83:4489–4497.
Article19. Ko EJ, Byon YY, Jee Y, Shin T, Park SC, Hahn TW, Joo HG. Maturation of bone marrow-derived dendritic cells by a novel β-glucan purified from Paenibacillus polymyxa JB115. J Vet Sci. 2011; 12:187–189.
Article20. Timmermans K, Plantinga TS, Kox M, Vaneker M, Scheffer GJ, Adema GJ, Joosten LA, Netea MG. Blueprints of signaling interactions between pattern recognition receptors: implications for the design of vaccine adjuvants. Clin Vaccine Immunol. 2013; 20:427–432.
Article21. Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007; 316:1628–1632.
Article22. Yamamoto M, Sato S, Hemmi H, Uematsu S, Hoshino K, Kaisho T, Takeuchi O, Takeda K, Akira S. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat Immunol. 2003; 4:1144–1150.
Article23. Kawai T, Akira S. TLR signaling. Semin Immunol. 2007; 19:24–32.
Article24. Hancock GE, Heers KM, Pryharski KS, Smith JD, Tiberio L. Adjuvants recognized by toll-like receptors inhibit the induction of polarized type 2 T cell responses by natural attachment (G) protein of respiratory syncytial virus. Vaccine. 2003; 21:4348–4358.
Article25. Prince GA, Mond JJ, Porter DD, Yim KC, Lan SJ, Klinman DM. Immunoprotective activity and safety of a respiratory syncytial virus vaccine: mucosal delivery of fusion glycoprotein with a CpG oligodeoxynucleotide adjuvant. J Virol. 2003; 77:13156–13160.
Article26. Didierlaurent AM, Morel S, Lockman L, Giannini SL, Bisteau M, Carlsen H, Kielland A, Vosters O, Vanderheyde N, Schiavetti F, et al. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J Immunol. 2009; 183:6186–6197.
Article27. Prince GA, Denamur F, Deschamps M, Garçon N, Prieels JP, Slaoui M, Thiriart C, Porter DD. Monophosphoryl lipid A adjuvant reverses a principal histologic parameter of formalin-inactivated respiratory syncytial virus vaccine-induced disease. Vaccine. 2001; 19:2048–2054.
Article28. Blanco JC, Boukhvalova MS, Pletneva LM, Shirey KA, Vogel SN. A recombinant anchorless respiratory syncytial virus (RSV) fusion (F) protein/monophosphoryl lipid A (MPL) vaccine protects against RSV-induced replication and lung pathology. Vaccine. 2014; 32:1495–1500.
Article29. Wack A, Baudner BC, Hilbert AK, Manini I, Nuti S, Tavarini S, Scheffczik H, Ugozzoli M, Singh M, Kazzaz J, et al. Combination adjuvants for the induction of potent, long-lasting antibody and T-cell responses to influenza vaccine in mice. Vaccine. 2008; 26:552–561.
Article30. Grossmann C, Tenbusch M, Nchinda G, Temchura V, Nabi G, Stone GW, Kornbluth RS, Uberla K. Enhancement of the priming efficacy of DNA vaccines encoding dendritic cell-targeted antigens by synergistic toll-like receptor ligands. BMC Immunol. 2009; 10:43.
Article31. Smith AM, Smith AP. A critical, nonlinear threshold dictates bacterial invasion and initial kinetics during influenza. Sci Rep. 2016; 6:38703.
Article32. Tate MD, Pickett DL, van Rooijen N, Brooks AG, Reading PC. Critical role of airway macrophages in modulating disease severity during influenza virus infection of mice. J Virol. 2010; 84:7569–7580.
Article33. Tate MD, Schilter HC, Brooks AG, Reading PC. Responses of mouse airway epithelial cells and alveolar macrophages to virulent and avirulent strains of influenza A virus. Viral Immunol. 2011; 24:77–88.
Article34. Liu Q, Zhou YH, Yang ZQ. The cytokine storm of severe influenza and development of immunomodulatory therapy. Cell Mol Immunol. 2016; 13:3–10.
Article35. Yokota S, Imagawa T, Miyamae T, Ito S, Nakajima S, Nezu A, Mori M. Hypothetical pathophysiology of acute encephalopathy and encephalitis related to influenza virus infection and hypothermia therapy. Pediatr Int. 2000; 42:197–203.
Article36. Russell JH, Ley TJ. Lymphocyte-mediated cytotoxicity. Annu Rev Immunol. 2002; 20:323–370.
Article37. Topham DJ, Tripp RA, Doherty PC. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J Immunol. 1997; 159:5197–5200.38. Hufford MM, Kim TS, Sun J, Braciale TJ. Antiviral CD8+ T cell effector activities in situ are regulated by target cell type. J Exp Med. 2011; 208:167–180.
Article39. Sun J, Madan R, Karp CL, Braciale TJ. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med. 2009; 15:277–284.
Article40. Hua L, Yao S, Pham D, Jiang L, Wright J, Sawant D, Dent AL, Braciale TJ, Kaplan MH, Sun J. Cytokine-dependent induction of CD4+ T cells with cytotoxic potential during influenza virus infection. J Virol. 2013; 87:11884–11893.
Article