Ann Dermatol.
2018 Feb;30(1):54-63. 10.5021/ad.2018.30.1.54.
Epidermal Growth Factor Attenuated the Expression of Inflammatory Cytokines in Human Epidermal Keratinocyte Exposed to Propionibacterium acnes
- Affiliations
-
- 1Life Science Research Institute, Daewoong Pharmaceutical Co., Ltd., Yongin, Korea.
- 2Department of Dermatology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. csesnumd@gmail.com
- KMID: 2399755
- DOI: http://doi.org/10.5021/ad.2018.30.1.54
Abstract
- BACKGROUND
While the beneficial effects of topical epidermal growth factor (EGF) on wound healing have been repeatedly reported, there are few reports about the effects of EGF on inflammatory skin diseases including acne.
OBJECTIVE
To clarify the effects of EGF on acne, it was investigated whether recombinant human EGF (rhEGF) signalling can affect Propionibacterium acnes-induced cytokine expression in human epidermal keratinocytes.
METHODS
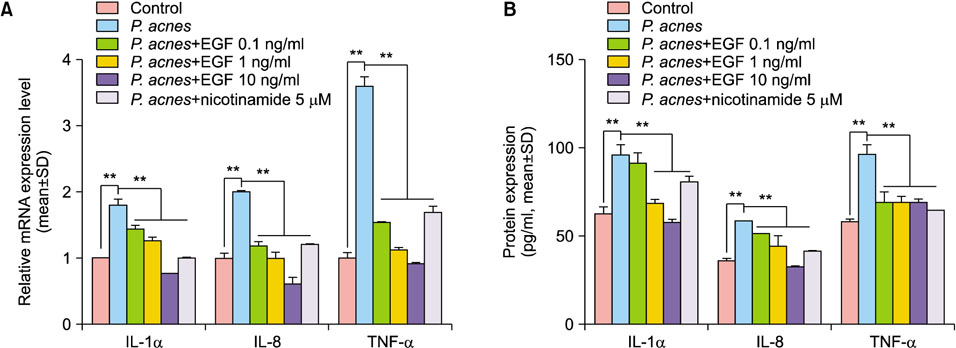
The cultured normal human epidermal keratinocytes (NHK) were co-treated with P. acnes and rhEGF, and mRNA levels of interleukin (IL)-1α, IL-8 and tumor necrosis factor (TNF)-α; toll-like receptor 2 (TLR2); and nuclear factor kappa B (NF-κB) were determined. Specific enzyme-linked immunosorbent assay kits were used to measure the IL-1α, IL-8 and TLR2 expression as well as the NF-κB activation in P. acnes and rhEGF-treated NHK. After infecting the cultured NHK with live P. acnes, an increased expression of IL-1α, IL-8 and TNF-α was detected, which was prevented by rhEGF co-treatment.
RESULTS
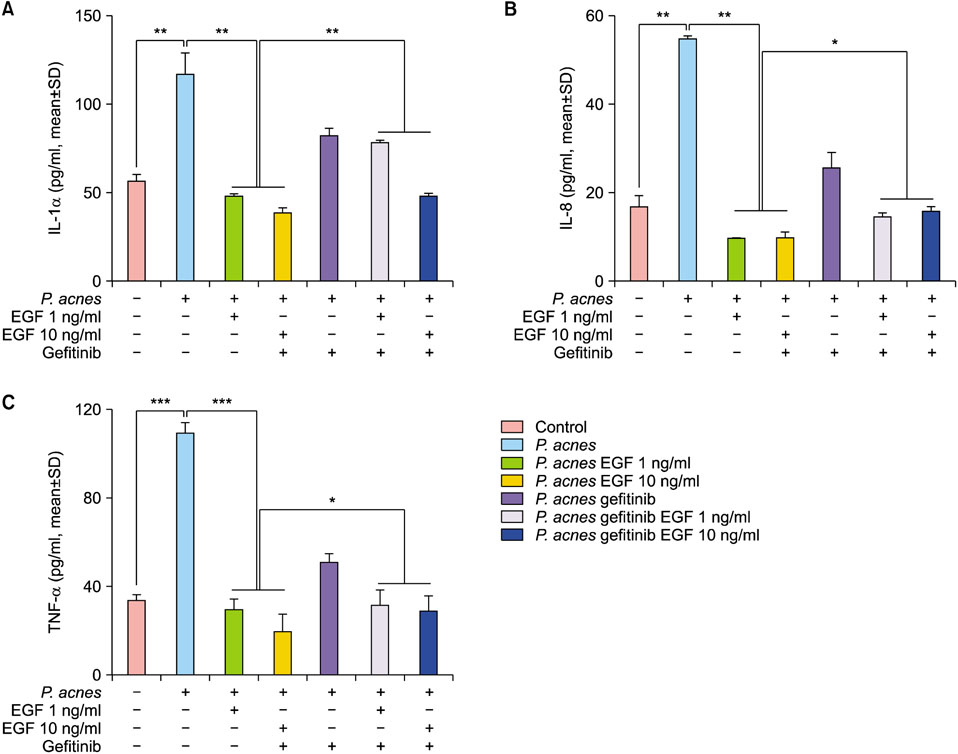
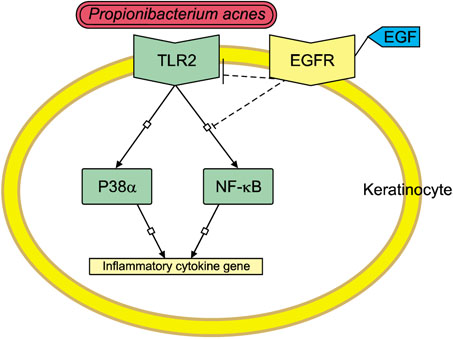
TLR2 and NF-κB activity increased after P. acnes treatment, and rhEGF treatment decreased TLR2 expression and NF-κB activity dose-dependently. The inhibition of EGF receptor by gefitinib attenuated the inhibitory effect of rhEGF on these increased expressions of proinflammatory cytokines and TLR2 and activity of NF-κB in NHK stimulated by P. acnes.
CONCLUSION
These results suggest that EGF attenuated P. acnes-induced inflammatory responses, at least in part, through the modulation of TLR2 signalling, and the topical application of rhEGF may be beneficial to relieve the inflammatory reactions of acne.
MeSH Terms
-
Acne Vulgaris
Cytokines*
Enzyme-Linked Immunosorbent Assay
Epidermal Growth Factor*
Humans*
Interleukin-8
Interleukins
Keratinocytes*
NF-kappa B
Propionibacterium acnes*
Propionibacterium*
Receptor, Epidermal Growth Factor
RNA, Messenger
Skin Diseases
Toll-Like Receptor 2
Tumor Necrosis Factor-alpha
Wound Healing
Cytokines
Epidermal Growth Factor
Interleukin-8
Interleukins
NF-kappa B
RNA, Messenger
Receptor, Epidermal Growth Factor
Toll-Like Receptor 2
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. McInturff JE, Modlin RL, Kim J. The role of toll-like receptors in the pathogenesis and treatment of dermatological disease. J Invest Dermatol. 2005; 125:1–8.
Article2. Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006; 124:783–801.
Article3. Shibata M, Katsuyama M, Onodera T, Ehama R, Hosoi J, Tagami H. Glucocorticoids enhance Toll-like receptor 2 expression in human keratinocytes stimulated with Propionibacterium acnes or proinflammatory cytokines. J Invest Dermatol. 2009; 129:375–382.
Article4. Li ZJ, Choi DK, Sohn KC, Seo MS, Lee HE, Lee Y, et al. Propionibacterium acnes activates the NLRP3 inflammasome in human sebocytes. J Invest Dermatol. 2014; 134:2747–2756.
Article5. Dispenza MC, Wolpert EB, Gilliland KL, Dai JP, Cong Z, Nelson AM, et al. Systemic isotretinoin therapy normalizes exaggerated TLR-2-mediated innate immune responses in acne patients. J Invest Dermatol. 2012; 132:2198–2205.
Article6. Selway JL, Kurczab T, Kealey T, Langlands K. Toll-like receptor 2 activation and comedogenesis: implications for the pathogenesis of acne. BMC Dermatol. 2013; 13:10.
Article7. Koff JL, Shao MX, Ueki IF, Nadel JA. Multiple TLRs activate EGFR via a signaling cascade to produce innate immune responses in airway epithelium. Am J Physiol Lung Cell Mol Physiol. 2008; 294:L1068–L1075.
Article8. Pastore S, Mascia F. Novel acquisitions on the immunoprotective roles of the EGF receptor in the skin. Expert Rev Dermatol. 2008; 3:525–527.
Article9. Wenczak BA, Lynch JB, Nanney LB. Epidermal growth factor receptor distribution in burn wounds. Implications for growth factor-mediated repair. J Clin Invest. 1992; 90:2392–2401.
Article10. Egger B, Carey HV, Procaccino F, Chai NN, Sandgren EP, Lakshmanan J, et al. Reduced susceptibility of mice overexpressing transforming growth factor alpha to dextran sodium sulphate induced colitis. Gut. 1998; 43:64–70.
Article11. Servold SA. Growth factor impact on wound healing. Clin Podiatr Med Surg. 1991; 8:937–953.12. Kim HK, Yeo IK, Li K, Kim BJ, Kim MN, Hong CK. Topical epidermal growth factor for the improvement of acne lesions: a randomized, double-blinded, placebo-controlled, split-face trial. Int J Dermatol. 2014; 53:1031–1036.
Article13. Grange PA, Raingeaud J, Calvez V, Dupin N. Nicotinamide inhibits Propionibacterium acnes-induced IL-8 production in keratinocytes through the NF-kappaB and MAPK pathways. J Dermatol Sci. 2009; 56:106–112.
Article14. Miller LS, Sørensen OE, Liu PT, Jalian HR, Eshtiaghpour D, Behmanesh BE, et al. TGF-alpha regulates TLR expression and function on epidermal keratinocytes. J Immunol. 2005; 174:6137–6143.
Article15. Bedford A, Chen T, Huynh E, Zhu C, Medeiros S, Wey D, et al. Epidermal growth factor containing culture supernatant enhances intestine development of early-weaned pigs in vivo: potential mechanisms involved. J Biotechnol. 2015; 196-197:9–19.
Article16. Mascia F, Mariani V, Girolomoni G, Pastore S. Blockade of the EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation. Am J Pathol. 2003; 163:303–312.
Article17. Pastore S, Mascia F, Mariotti F, Dattilo C, Mariani V, Girolomoni G. ERK1/2 regulates epidermal chemokine expression and skin inflammation. J Immunol. 2005; 174:5047–5056.
Article18. Valins W, Amini S, Berman B. The expression of toll-like receptors in dermatological diseases and the therapeutic effect of current and newer topical toll-like receptor modulators. J Clin Aesthet Dermatol. 2010; 3:20–29.19. West AP, Koblansky AA, Ghosh S. Recognition and signaling by toll-like receptors. Annu Rev Cell Dev Biol. 2006; 22:409–437.
Article20. Pietrocola G, Arciola CR, Rindi S, Di Poto A, Missineo A, Montanaro L, et al. Toll-like receptors (TLRs) in innate immune defense against Staphylococcus aureus. Int J Artif Organs. 2011; 34:799–810.
Article21. Kim J, Ochoa MT, Krutzik SR, Takeuchi O, Uematsu S, Legaspi AJ, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002; 169:1535–1541.
Article22. Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004; 25:280–288.
Article23. Kim H, Moon SY, Sohn MY, Lee WJ. Insulin-like growth factor-1 increases the expression of inflammatory biomarkers and sebum production in cultured sebocytes. Ann Dermatol. 2017; 29:20–25.
Article24. An H, Yu Y, Zhang M, Xu H, Qi R, Yan X, et al. Involvement of ERK, p38 and NF-kappaB signal transduction in regulation of TLR2, TLR4 and TLR9 gene expression induced by lipopolysaccharide in mouse dendritic cells. Immunology. 2002; 106:38–45.
Article25. Anderson KV. Toll signaling pathways in the innate immune response. Curr Opin Immunol. 2000; 12:13–19.
Article26. Zhang G, Ghosh S. Toll-like receptor-mediated NF-kappaB activation: a phylogenetically conserved paradigm in innate immunity. J Clin Invest. 2001; 107:13–19.
Article27. Mendelsohn J, Baselga J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol. 2003; 21:2787–2799.
Article28. Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007; 7:454–465.
Article29. Cooney RN. Suppressors of cytokine signaling (SOCS): inhibitors of the JAK/STAT pathway. Shock. 2002; 17:83–90.
Article30. Arakawa S, Hatano Y, Katagiri K. Differential expression of mRNA for Th1 and Th2 cytokine-associated transcription factors and suppressors of cytokine signalling in peripheral blood mononuclear cells of patients with atopic dermatitis. Clin Exp Immunol. 2004; 135:505–510.
Article31. Federici M, Giustizieri ML, Scarponi C, Girolomoni G, Albanesi C. Impaired IFN-gamma-dependent inflammatory responses in human keratinocytes overexpressing the suppressor of cytokine signaling 1. J Immunol. 2002; 169:434–442.
Article32. Goren I, Linke A, Müller E, Pfeilschifter J, Frank S. The suppressor of cytokine signaling-3 is upregulated in impaired skin repair: implications for keratinocyte proliferation. J Invest Dermatol. 2006; 126:477–485.
Article33. Shostak K, Chariot A. EGFR and NF-κB: partners in cancer. Trends Mol Med. 2015; 21:385–393.
Article34. Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009; 9:463–475.
Article35. Hu JC, Sadeghi P, Pinter-Brown LC, Yashar S, Chiu MW. Cutaneous side effects of epidermal growth factor receptor inhibitors: clinical presentation, pathogenesis, and management. J Am Acad Dermatol. 2007; 56:317–326.
Article36. Lichtenberger BM, Gerber PA, Holcmann M, Buhren BA, Amberg N, Smolle V, et al. Epidermal EGFR controls cutaneous host defense and prevents inflammation. Sci Transl Med. 2013; 5:199ra111.
Article37. Park K, Ommori R, Imoto K, Asada H. Epidermal growth factor receptor inhibitors selectively inhibit the expressions of human β-defensins induced by Staphylococcus epidermidis. J Dermatol Sci. 2014; 75:94–99.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Blue Light on Propionibacterium acnes and the Expression of Inflammatory Cytokines in HaCaT Cells
- Prognostic significance of epidermal growth factor receptor expression in human gastric carcinoma
- The Expression of Keratinocyte Growth Factor mRNA in Dendritic Epidermal T Cell
- Epidermal growth factor receptors increase in rabbit embryonal implantation
- Correlation of epidermal growth factor receptor expression with prognostic factors in patients with ovarian neoplasms