Effects of Next-Generation Low-Energy Extracorporeal Shockwave Therapy on Erectile Dysfunction in an Animal Model of Diabetes
- Affiliations
-
- 1Department of Urology, The Catholic University of Korea, Seoul St. Mary's Hospital, Seoul, Korea. ksw1227@catholic.ac.kr
- 2Department of Urology, The Catholic University of Korea, Incheon St. Mary's Hospital, Incheon, Korea.
- 3Catholic Integrative Medicine Research Institute, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 4Department of Urology, Korea University College of Medicine, Seoul, Korea.
- KMID: 2398568
- DOI: http://doi.org/10.5534/wjmh.17024
Abstract
- PURPOSE
Gene therapy, stem cell therapy, and low-energy extracorporeal shockwave therapy (ESWT) have been investigated as treatments for refractory erectile dysfunction (ED), but inconclusive evidence has been obtained. We investigated the effect of a next-generation electromagnetic cylinder ESWT device on an animal model of ED.
MATERIALS AND METHODS
Diabetes mellitus (DM)-induced rats were divided into 3 groups: group 1, control; group 2, DM; and group 3, DM+ESWT. Rats were treated with ESWT 3 times a week for 2 weeks. After the treatment course, intracavernous pressure was measured and the corpus cavernosum and cavernous nerve were evaluated.
RESULTS
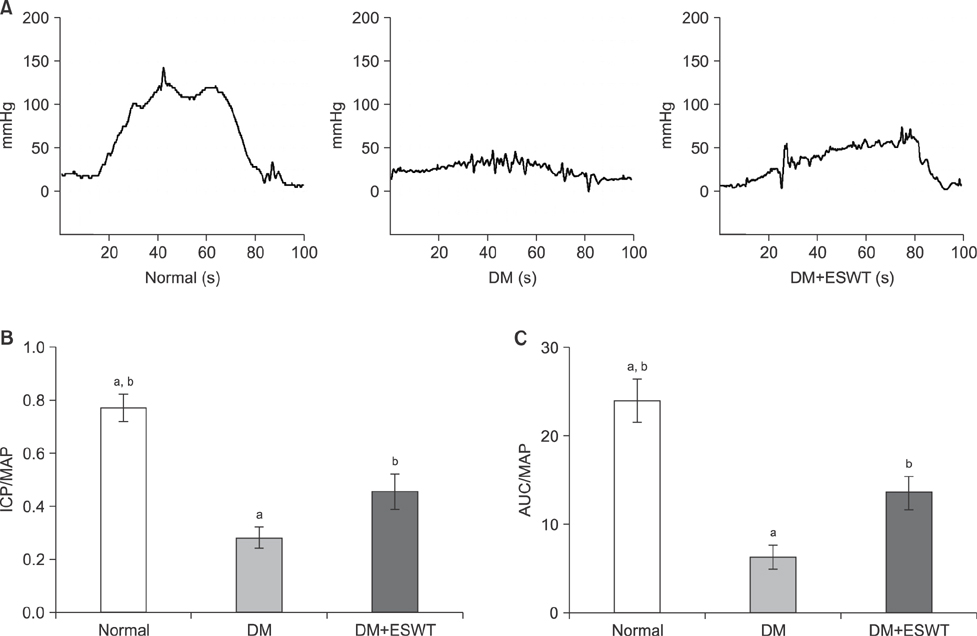
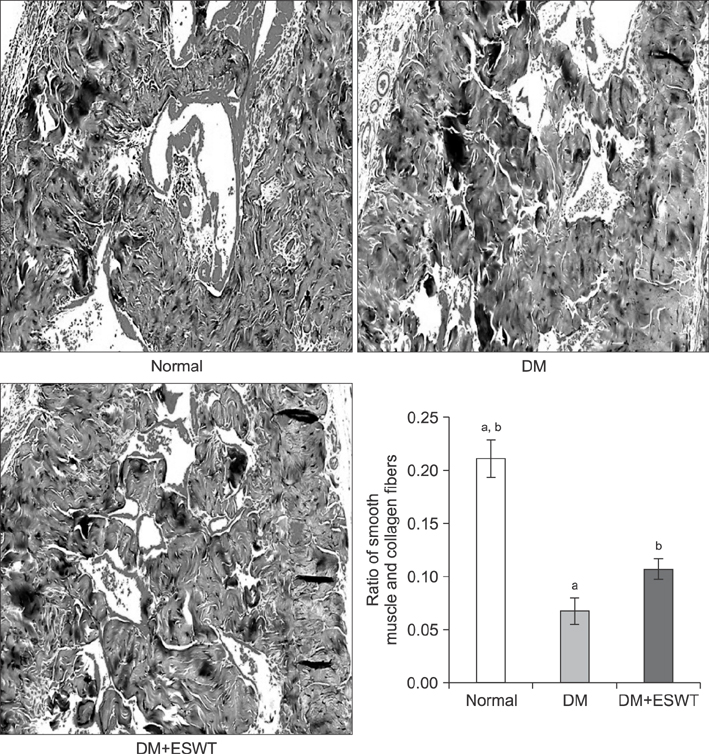
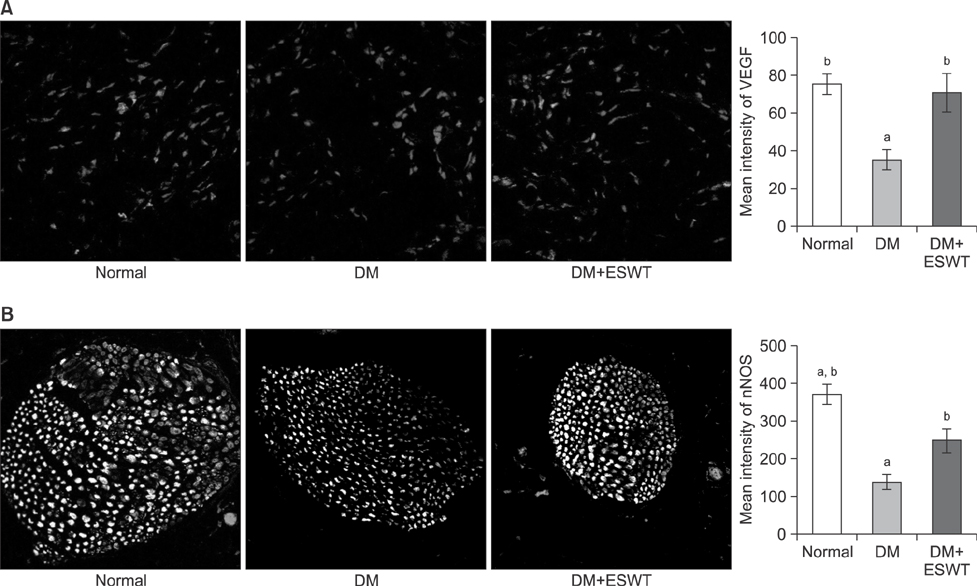
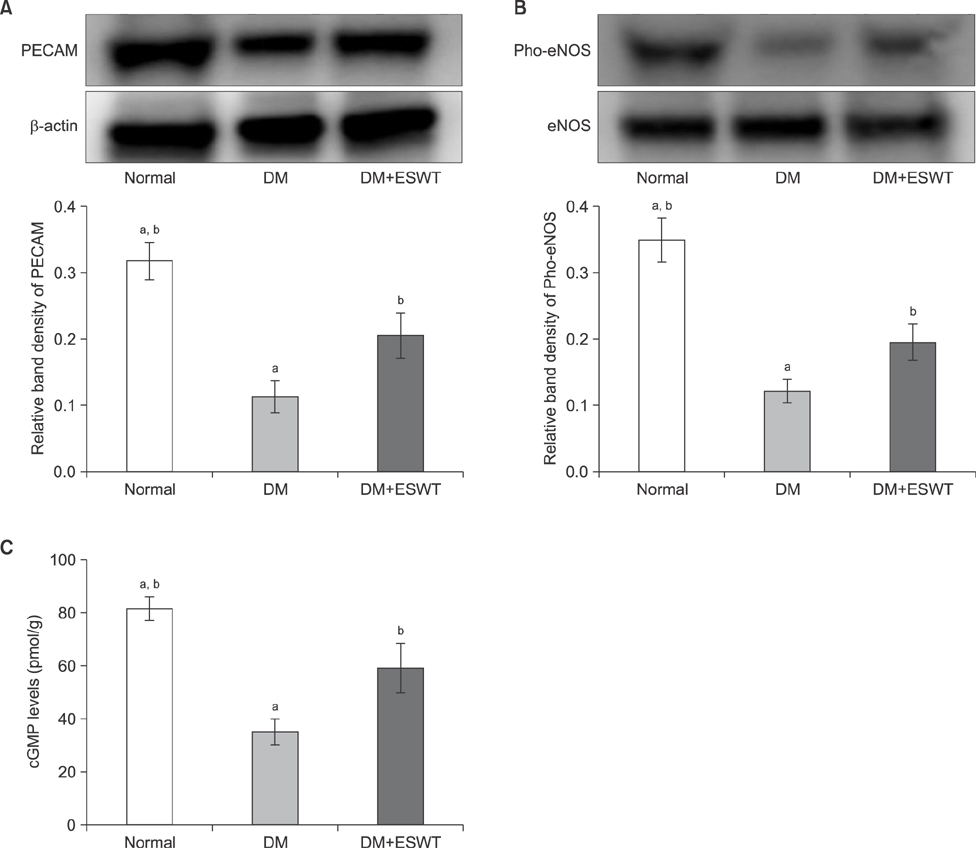
In the DM group, all parameters predicted to be significantly lower in the ED model had statistically significantly decreased (p < 0.01). As a measurement of erectile function, intracavernous pressure was evaluated. The DM+ESWT group exhibited significantly restored erectile function compared to the DM group (p < 0.05). Moreover, ESWT treatment restored smooth muscle content, as assessed by Masson's trichrome staining (p < 0.05). Finally, corporal tissue and the dorsal nerve were evaluated by immunohistochemistry, Western blotting, and ELISA. After ESWT treatment, vascular endothelial growth factor (VEGF), endothelial nitric oxide synthase (eNOS), platelet endothelial cell adhesion molecule-1, cyclic guanosine monophosphate, and neuronal nitric oxide synthase (nNOS) expression levels were restored to levels in the DM group (p < 0.05).
CONCLUSIONS
Electromagnetic cylinder ESWT device resulted in increased VEGF, nNOS, and eNOS expression; reduced smooth muscle atrophy; and increased endothelial cell regeneration in a DM-associated ED model. Our data suggest that safe and effective application could be possible in future clinical studies.
MeSH Terms
-
Animals*
Antigens, CD31
Atrophy
Blotting, Western
Diabetes Mellitus
Endothelial Cells
Enzyme-Linked Immunosorbent Assay
Erectile Dysfunction*
Genetic Therapy
Guanosine Monophosphate
Immunohistochemistry
Magnets
Male
Models, Animal*
Muscle, Smooth
Nitric Oxide Synthase Type I
Nitric Oxide Synthase Type III
Rats
Regeneration
Stem Cells
Vascular Endothelial Growth Factor A
Antigens, CD31
Guanosine Monophosphate
Nitric Oxide Synthase Type I
Nitric Oxide Synthase Type III
Vascular Endothelial Growth Factor A
Figure
Cited by 2 articles
-
Synergistic effects of extracorporeal shockwave therapy and modified Ojayeonjonghwan on erectile dysfunction in an animal model of diabetes
Hyun Cheol Jeong, Woong Jin Bae, Guan Qun Zhu, Seung Hwan Jeon, Sae Woong Choi, Su Jin Kim, Hyuk Jin Cho, Sung-Hoo Hong, Ji Youl Lee, Sung Yeoun Hwang, Sae Woong Kim
Investig Clin Urol. 2019;60(4):285-294. doi: 10.4111/icu.2019.60.4.285.Electromagnetic Low-Intensity Extracorporeal Shock Wave Therapy in Patients with Erectile Dysfunction: A Sham-Controlled, Double-Blind, Randomized Prospective Study
Kang Sup Kim, Hyun Cheol Jeong, Sae Woong Choi, Yong Sun Choi, Hyuk Jin Cho, U-Syn Ha, Sung-Hoo Hong, Ji Youl Lee, Seung Wook Lee, Sun Tae Ahn, Du Geon Moon, Woong Jin Bae, Sae Woong Kim
World J Mens Health. 2020;38(2):236-242. doi: 10.5534/wjmh.190130.
Reference
-
1. Montorsi F, Adaikan G, Becher E, Giuliano F, Khoury S, Lue TF, et al. Summary of the recommendations on sexual dysfunctions in men. J Sex Med. 2010; 7:3572–3588.
Article2. Litwin MS, Nied RJ, Dhanani N. Health-related quality of life in men with erectile dysfunction. J Gen Intern Med. 1998; 13:159–166.
Article3. Dorsey P, Keel C, Klavens M, Hellstrom WJ. Phosphodiesterase type 5 (PDE5) inhibitors for the treatment of erectile dysfunction. Expert Opin Pharmacother. 2010; 11:1109–1122.
Article4. Melman A, Bar-Chama N, McCullough A, Davies K, Christ G. hMaxi-K gene transfer in males with erectile dysfunction: results of the first human trial. Hum Gene Ther. 2006; 17:1165–1176.
Article5. Deng W, Bivalacqua TJ, Hellstrom WJ, Kadowitz PJ. Gene and stem cell therapy for erectile dysfunction. Int J Impot Res. 2005; 17:Suppl 1. S57–S63.
Article6. Chaussy C, Brendel W, Schmiedt E. Extracorporeally induced destruction of kidney stones by shock waves. Lancet. 1980; 2:1265–1268.
Article7. Chaussy C, Schmiedt E. Shock wave treatment for stones in the upper urinary tract. Urol Clin North Am. 1983; 10:743–750.
Article8. Chaussy C, Schmiedt E, Jocham D, Brendel W, Forssmann B, Walther V. First clinical experience with extracorporeally induced destruction of kidney stones by shock waves. J Urol. 1982; 127:417–420.
Article9. Young SR, Dyson M. The effect of therapeutic ultrasound on angiogenesis. Ultrasound Med Biol. 1990; 16:261–269.
Article10. Wang CJ. An overview of shock wave therapy in musculoskeletal disorders. Chang Gung Med J. 2003; 26:220–232.11. Nurzynska D, Di Meglio F, Castaldo C, Arcucci A, Marlinghaus E, Russo S, et al. Shock waves activate in vitro cultured progenitors and precursors of cardiac cell lineages from the human heart. Ultrasound Med Biol. 2008; 34:334–342.
Article12. Qiu X, Lin G, Xin Z, Ferretti L, Zhang H, Lue TF, et al. Effects of low-energy shockwave therapy on the erectile function and tissue of a diabetic rat model. J Sex Med. 2013; 10:738–746.
Article13. Vardi Y, Appel B, Kilchevsky A, Gruenwald I. Does low intensity extracorporeal shock wave therapy have a physiological effect on erectile function? Short-term results of a randomized, double-blind, sham controlled study. J Urol. 2012; 187:1769–1775.
Article14. Bickenbach JR, Chism E. Selection and extended growth of murine epidermal stem cells in culture. Exp Cell Res. 1998; 244:184–195.
Article15. Lee MC, El-Sakka AI, Graziottin TM, Ho HC, Lin CS, Lue TF. The effect of vascular endothelial growth factor on a rat model of traumatic arteriogenic erectile dysfunction. J Urol. 2002; 167:761–767.
Article16. Lin CS, Xin ZC, Wang Z, Deng C, Huang YC, Lin G, et al. Stem cell therapy for erectile dysfunction: a critical review. Stem Cells Dev. 2012; 21:343–351.
Article17. Ogden JA, Tóth-Kischkat A, Schultheiss R. Principles of shock wave therapy. Clin Orthop Relat Res. 2001; (387):8–17.
Article18. Onose G, Daia Chendreanu C, Haras M, Spinu A, Andone I. Extracorporeal shock wave therapy: a new “wave” (also) in physiatry? Practica Medicala. 2011; 6:35–42.19. Park K, Ahn KY, Kim MK, Lee SE, Kang TW, Ryu SB. Intracavernosal injection of vascular endothelial growth factor improves erectile function in aged rats. Eur Urol. 2004; 46:403–407.
Article20. Yamanaka M, Shirai M, Shiina H, Tanaka Y, Enokida H, Tsujimura A, et al. Vascular endothelial growth factor restores erectile function through inhibition of apoptosis in diabetic rat penile crura. J Urol. 2005; 173:318–323.
Article21. Lee NH, Seo CS, Lee HY, Jung DY, Lee JK, Lee JA, et al. Hepatoprotective and antioxidative activities of cornus officinalis against acetaminophen-induced hepatotoxicity in mice. Evid Based Complement Alternat Med. 2012; DOI: 10.1155/2012/804924.22. Wang W, Xu J, Li L, Wang P, Ji X, Ai H, et al. Neuroprotective effect of morroniside on focal cerebral ischemia in rats. Brain Res Bull. 2010; 83:196–201.
Article23. Melis MR, Succu S, Mauri A, Argiolas A. Nitric oxide production is increased in the paraventricular nucleus of the hypothalamus of male rats during non-contact penile erections and copulation. Eur J Neurosci. 1998; 10:1968–1974.
Article24. Dashwood MR, Crump A, Shi-Wen X, Loesch A. Identification of neuronal nitric oxide synthase (nNOS) in human penis: a potential role of reduced neuronally-derived nitric oxide in erectile dysfunction. Curr Pharm Biotechnol. 2011; 12:1316–1321.25. Thorve VS, Kshirsagar AD, Vyawahare NS, Joshi VS, Ingale KG, Mohite RJ. Diabetes-induced erectile dysfunction: epidemiology, pathophysiology and management. J Diabetes Complications. 2011; 25:129–136.
Article26. Zhou F, Xin H, Liu T, Li GY, Gao ZZ, Liu J, et al. Effects of icariside II on improving erectile function in rats with streptozotocin-induced diabetes. J Androl. 2012; 33:832–844.
Article27. Albersen M, Lin G, Fandel TM, Zhang H, Qiu X, Lin CS, et al. Functional, metabolic, and morphologic characteristics of a novel rat model of type 2 diabetes-associated erectile dysfunction. Urology. 2011; 78:476476.e1–476.e8.
Article28. Kwon MH, Ryu JK, Kim WJ, Jin HR, Song KM, Kwon KD, et al. Effect of intracavernous administration of angiopoietin-4 on erectile function in the streptozotocin-induced diabetic mouse. J Sex Med. 2013; 10:2912–2927.
Article29. Bhojani N, Mandeville JA, Hameed TA, Soergel TM, McAteer JA, Williams JC Jr, et al. Lithotripter outcomes in a community practice setting: comparison of an electromagnetic and an electrohydraulic lithotripter. J Urol. 2015; 193:875–879.
Article30. Mustafa M, Aburas H, Helo FM, Qarawi L. Electromagnetic and electrohydraulic shock wave lithotripsy-induced urothelial damage: is there a difference? J Endourol. 2017; 31:180–184.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Prospective, Randomized, Double-Blinded, Clinical Trial Using a Second-Generation Duolith SD1 Low-Intensity Shockwave Machine in Males with Vascular Erectile Dysfunction
- Low-Intensity Shock Wave Therapy and Its Application to Erectile Dysfunction
- Regenerative therapies as a potential treatment of erectile dysfunction
- Synergistic effects of extracorporeal shockwave therapy and modified Ojayeonjonghwan on erectile dysfunction in an animal model of diabetes
- Low-intensity extracorporeal shock wave therapy for erectile dysfunction: Myths and realities