Korean J Physiol Pharmacol.
2017 Nov;21(6):695-702. 10.4196/kjpp.2017.21.6.695.
Developmental changes in GABAA tonic inhibition are compromised by multiple mechanisms in preadolescent dentate gyrus granule cells
- Affiliations
-
- 1Department of Physiology, School of Medicine and Brain Research Institute, Chungnam National University, Daejeon 35015, Korea. jinbong@cnu.ac.kr
- KMID: 2395265
- DOI: http://doi.org/10.4196/kjpp.2017.21.6.695
Abstract
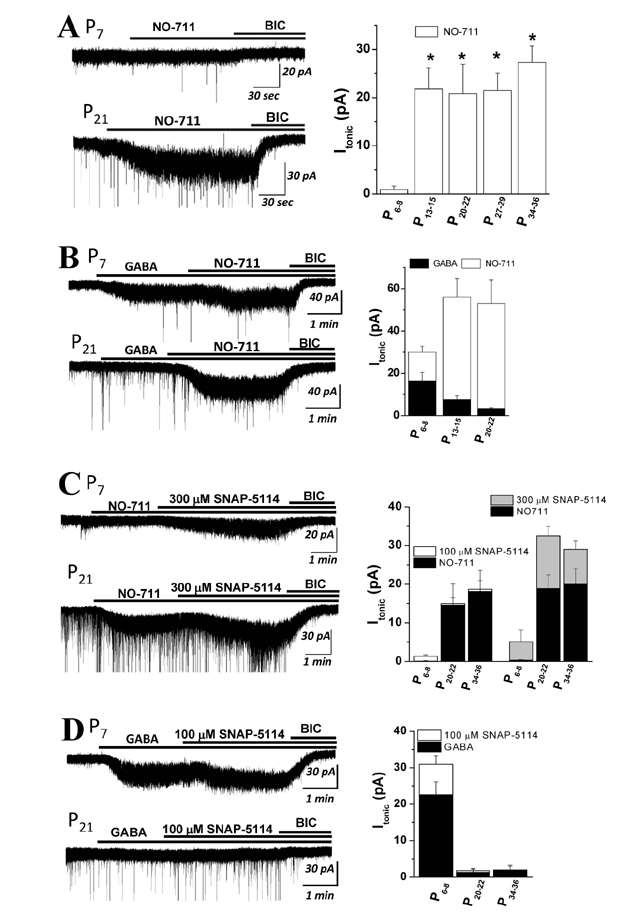
- The sustained tonic currents (I(tonic)) generated by γ-aminobutyric acid A receptors (GABA(A)Rs) are implicated in diverse age-dependent brain functions. While various mechanisms regulating I(tonic) in the hippocampus are known, their combined role in I(tonic) regulation is not well understood in different age groups. In this study, we demonstrated that a developmental increase in GABA transporter (GAT) expression, combined with gradual decrease in GABA(A)R α₅ subunit, resulted in various I(tonic) in the dentate gyrus granule cells (DGGCs) of preadolescent rats. Both GAT-1 and GAT-3 expression gradually increased at infantile (P₆₋₈ and Pâ‚₃₋â‚â‚…) and juvenile (P₂₀₋₂₂ and P₂₇₋₂₉) stages, with stabilization observed thereafter in adolescents (P₃₄₋₃₆) and young adults (Pâ‚„â‚₋₄₃). I(tonic) facilitation of a selective GAT-1 blocker (NO-711) was significantly less at P₆₋₈ than after Pâ‚₃₋â‚â‚…. The facilitation of I(tonic) by SNAP-5114, a GAT-3 inhibitor, was negligible in the absence of exogenous GABA at all tested ages. In contrast, I(tonic) in the presence of a nonselective GAT blocker (nipecotic acid, NPA) gradually decreased with age during the preadolescent period, which was mimicked by I(tonic) changes in the presence of exogenous GABA. I(tonic) sensitivity to L-655,708, a GABA(A)R α₅ subunit inverse agonist, gradually decreased during the preadolescent period in the presence of NPA or exogenous GABA. Finally, Western blot analysis showed that the expression of the GABA(A)R α₅ subunit in the dentate gyrus gradually decreased with age. Collectively, our results suggested that the I(tonic) regulation of altered GATs is under the final tune of GABA(A)R α₅ subunit activation in DGGCs at different ages.
MeSH Terms
Figure
Reference
-
1. Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005; 6:215–229.2. Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABA A receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004; 27:262–269.3. Brickley SG, Mody I. Extrasynaptic GABA(A) receptors: their function in the CNS and implications for disease. Neuron. 2012; 73:23–34.4. Hines RM, Davies PA, Moss SJ, Maguire J. Functional regulation of GABAA receptors in nervous system pathologies. Curr Opin Neurobiol. 2012; 22:552–558.5. Jang HJ, Cho KH, Park SW, Kim MJ, Yoon SH, Rhie DJ. The development of phasic and tonic inhibition in the rat visual cortex. Korean J Physiol Pharmacol. 2010; 14:399–405.6. Pandit S, Jeong JA, Jo JY, Cho HS, Kim DW, Kim JM, Ryu PD, Lee SY, Kim HW, Jeon BH, Park JB. Dual mechanisms diminishing tonic GABAA inhibition of dentate gyrus granule cells in Noda epileptic rats. J Neurophysiol. 2013; 110:95–102.7. Pandit S, Jo JY, Lee SU, Lee YJ, Lee SY, Ryu PD, Lee JU, Kim HW, Jeon BH, Park JB. Enhanced astroglial GABA uptake attenuates tonic GABAA inhibition of the presympathetic hypothalamic paraventricular nucleus neurons in heart failure. J Neurophysiol. 2015; 114:914–926.8. Semyanov A, Walker MC, Kullmann DM. GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nat Neurosci. 2003; 6:484–490.9. Scimemi A, Semyanov A, Sperk G, Kullmann DM, Walker MC. Multiple and plastic receptors mediate tonic GABAA receptor currents in the hippocampus. J Neurosci. 2005; 25:10016–10024.10. Holter NI, Zylla MM, Zuber N, Bruehl C, Draguhn A. Tonic GABAergic control of mouse dentate granule cells during postnatal development. Eur J Neurosci. 2010; 32:1300–1309.11. Lee CY, Liou HH. GABAergic tonic inhibition is regulated by developmental age and epilepsy in the dentate gyrus. Neuroreport. 2013; 24:515–519.12. Fleming RL, Wilson WA, Swartzwelder HS. Magnitude and ethanol sensitivity of tonic GABAA receptor-mediated inhibition in dentate gyrus changes from adolescence to adulthood. J Neurophysiol. 2007; 97:3806–3811.13. Bright DP, Smart TG. Methods for recording and measuring tonic GABAA receptor-mediated inhibition. Front Neural Circuits. 2013; 7:193.14. Borden LA. GABA transporter heterogeneity: pharmacology and cellular localization. Neurochem Int. 1996; 29:335–356.15. Song I, Volynski K, Brenner T, Ushkaryov Y, Walker M, Semyanov A. Different transporter systems regulate extracellular GABA from vesicular and non-vesicular sources. Front Cell Neurosci. 2013; 7:23.16. Kersanté F, Rowley SC, Pavlov I, Gutièrrez-Mecinas M, Semyanov A, Reul JM, Walker MC, Linthorst AC. A functional role for both-aminobutyric acid (GABA) transporter-1 and GABA transporter-3 in the modulation of extracellular GABA and GABAergic tonic conductances in the rat hippocampus. J Physiol. 2013; 591:2429–2441.17. Evans JE, Frostholm A, Rotter A. Embryonic and postnatal expression of four gamma-aminobutyric acid transporter mRNAs in the mouse brain and leptomeninges. J Comp Neurol. 1996; 376:431–446.18. Pai YH, Lim CS, Park KA, Cho HS, Lee GS, Shin YS, Kim HW, Jeon BH, Yoon SH, Park JB. Facilitation of AMPA receptor-mediated steady-state current by extrasynaptic NMDA receptors in supraoptic magnocellular neurosecretory cells. Korean J Physiol Pharmacol. 2016; 20:425–432.19. Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004; 429:184–187.20. Suzdak PD, Frederiksen K, Andersen KE, Sørensen PO, Knutsen LJ, Nielsen EB. NNC-711, a novel potent and selective gamma-aminobutyric acid uptake inhibitor: pharmacological characterization. Eur J Pharmacol. 1992; 224:189–198.21. Quirk K, Blurton P, Fletcher S, Leeson P, Tang F, Mellilo D, Ragan CI, McKernan RM. [3H]L-655,708, a novel ligand selective for the benzodiazepine site of GABAA receptors which contain the alpha 5 subunit. Neuropharmacology. 1996; 35:1331–1335.22. Gao H, Smith BN. Tonic GABAA receptor-mediated inhibition in the rat dorsal motor nucleus of the vagus. J Neurophysiol. 2010; 103:904–914.23. Keros S, Hablitz JJ. Subtype-specific GABA transporter antagonists synergistically modulate phasic and tonic GABAA conductances in rat neocortex. J Neurophysiol. 2005; 94:2073–2085.24. Solís JM, Nicoll RA. Postsynaptic action of endogenous GABA released by nipecotic acid in the hippocampus. Neurosci Lett. 1992; 147:16–20.25. Demarque M, Represa A, Becq H, Khalilov I, Ben-Ari Y, Aniksztejn L. Paracrine intercellular communication by a Ca2+- and SNARE-independent release of GABA and glutamate prior to synapse formation. Neuron. 2002; 36:1051–1061.26. Taylor J, Gordon-Weeks PR. Calcium-independent gamma-aminobutyric acid release from growth cones: role of gamma-aminobutyric acid transport. J Neurochem. 1991; 56:273–280.27. Richerson GB, Wu Y. Dynamic equilibrium of neurotransmitter transporters: not just for reuptake anymore. J Neurophysiol. 2003; 90:1363–1374.28. Glykys J, Mann EO, Mody I. Which GABA(A) receptor subunits are necessary for tonic inhibition in the hippocampus? J Neurosci. 2008; 28:1421–1426.29. Barrett-Jolley R. Nipecotic acid directly activates GABA(A)-like ion channels. Br J Pharmacol. 2001; 133:673–678.30. Gill KM, Grace AA. The role of α5 GABAA receptor agonists in the treatment of cognitive deficits in schizophrenia. Curr Pharm Des. 2014; 20:5069–5076.31. Prut L, Prenosil G, Willadt S, Vogt K, Fritschy JM, Crestani F. A reduction in hippocampal GABAA receptor alpha5 subunits disrupts the memory for location of objects in mice. Genes Brain Behav. 2010; 9:478–488.32. Atack JR. Preclinical and clinical pharmacology of the GABAA receptor alpha5 subtype-selective inverse agonist alpha5IA. Pharmacol Ther. 2010; 125:11–26.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of acute pentylenetetrazol injection induced epileptic seizures on rat dentate gyrus at different postnatal ages
- Temporal Change of Calbindin-D28k Immunoreactivity in the Dentate Gyrus of Voluntary Running Mouse
- Calcium Influx is Responsible for Afterdepolarizations in Rat Hippocampal Dentate Granule Cells
- The Effects of Repeated Restraint Stress on the Synaptic Plasticity in the Inner Molecular Layer of Mouse Dentate Gyrus
- Postnatal changes in glucose transporter 3 expression in the dentate gyrus of the C57BL/6 mouse model