Lab Anim Res.
2016 Mar;32(1):1-7. 10.5625/lar.2016.32.1.1.
Postnatal changes in glucose transporter 3 expression in the dentate gyrus of the C57BL/6 mouse model
- Affiliations
-
- 1Department of Anatomy and Cell Biology, College of Veterinary Medicine, and Research Institute for Veterinary Science, Seoul National University, Seoul 08826, Korea. vetmed2@snu.ac.kr
- 2Department of Biochemistry and Molecular Biology, Research Institute of Oral Sciences, College of Dentistry, Kangneung-Wonju National University, Gangneung 25457, Korea.
- 3Department of Veterinary Internal Medicine and Geriatrics, College of Veterinary Medicine, Kangwon National University, Chuncheon 24341, Korea.
- 4KMPC (Korea Mouse Phenotyping Center), Seoul National University, Seoul 08826, Korea.
- KMID: 2312148
- DOI: http://doi.org/10.5625/lar.2016.32.1.1
Abstract
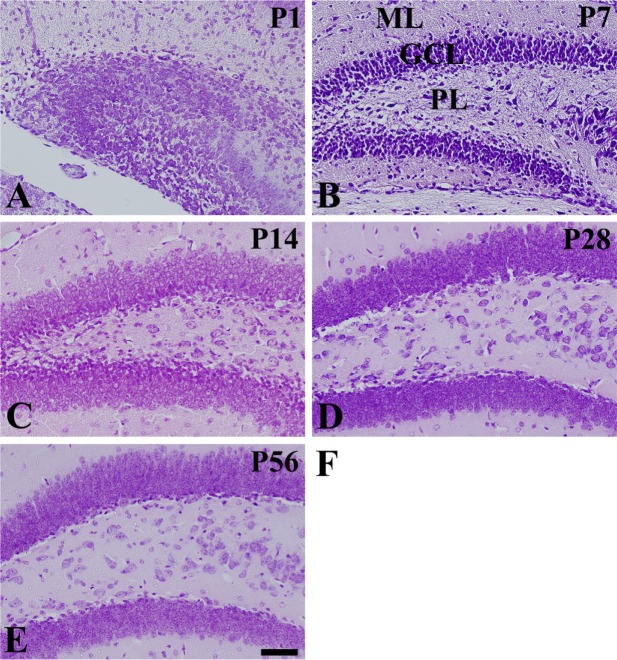
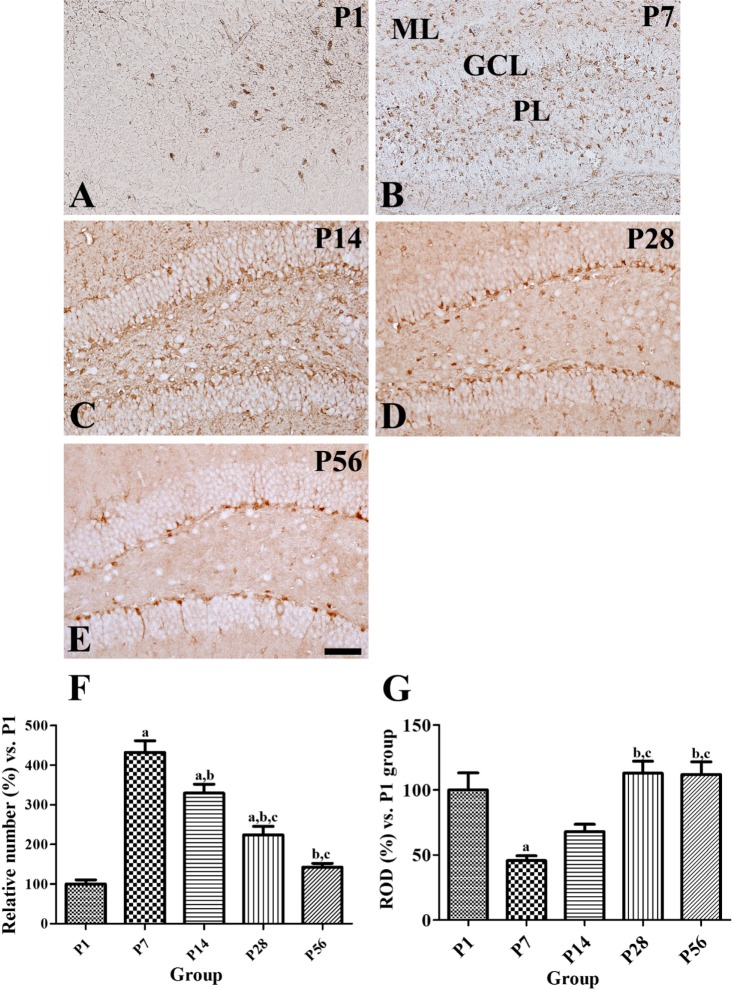
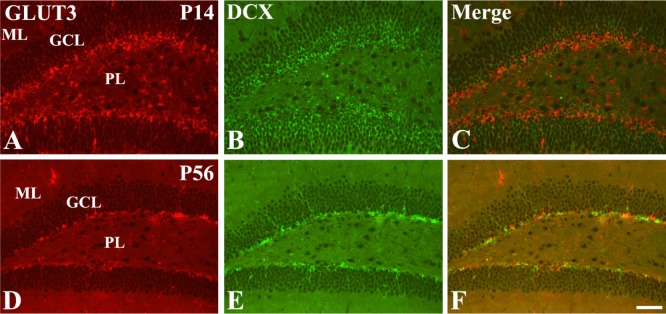
- In this study, we observed the ontogenetic changes in glucose transporter 3 (GLUT3) immunoreactivity, a major neuronal GLUT, in the dentate gyrus of mouse brains at various ages: postnatal day (P) 1, 7, 14, 28, and 56. At P1, cresyl violet staining showed abundant neurons in the dentate gyrus, whereas the granule cell layer was ill-defined. At P7, the granule cell layer was observed, and cresyl violet-positive cells were dispersed throughout the polymorphic layer. At P14, the granule cell layer was well-defined, and cresyl violet positive cells were detected abundantly in the polymorphic layer. At P28 and P56, cresyl violet-positive cells were observed in the granule cell layer, as well as in the polymorphic layer. At P1, GLUT3 immunoreactivity was detected in the dentate gyrus. At P7, GLUT3 immunoreactive cells were scattered in the polymorphic and molecular layer. However, at P14, GLUT3 immunoreactivity was observed in the polymorphic layer as well as subgranular zone of the dentate gyrus. At P28, GLUT3 immunoreactivity was detected in the polymorphic layer of the dentate gyrus. At P56, GLUT3 immunoreactivity was observed predominantly in the subgranular zone of the dentate gyrus. GLUT3 immunoreactive cells were mainly colocalized with doublecortin, which is a marker for differentiated neuroblasts, in the polymorphic layer and subgranular zone of dentate gyrus at P14 and P56. These results suggest that the expression of GLUT3 is closely associated with postnatal development of the dentate gyrus and adult neurogenesis.
MeSH Terms
Figure
Cited by 1 articles
-
Decrease in glucose transporter 1 levels and translocation of glucose transporter 3 in the dentate gyrus of C57BL/6 mice and gerbils with aging
Kwon Young Lee, Dae Young Yoo, Hyo Young Jung, Loktam Baek, Hangyul Lee, Hyun Jung Kwon, Jin Young Chung, Seok Hoon Kang, Dae Won Kim, In Koo Hwang, Jung Hoon Choi
Lab Anim Res. 2018;34(2):58-64. doi: 10.5625/lar.2018.34.2.58.
Reference
-
1. Pellegrini M, Mansouri A, Simeone A, Boncinelli E, Gruss P. Dentate gyrus formation requires Emx2. Development. 1996; 122(12):3893–3898. PMID: 9012509.
Article2. Bayer SA. Development of the hippocampal region in the rat. II. Morphogenesis during embryonic and early postnatal life. J Comp Neurol. 1980; 190(1):115–134. PMID: 7381049.
Article3. Schlessinger AR, Cowan WM, Gottlieb DI. An autoradiographic study of the time of origin and the pattern of granule cell migration in the dentate gyrus of the rat. J Comp Neurol. 1975; 159(2):149–175. PMID: 1112911.
Article4. Ngwenya LB, Heyworth NC, Shwe Y, Moore TL, Rosene DL. Age-related changes in dentate gyrus cell numbers, neurogenesis, and associations with cognitive impairments in the rhesus monkey. Front Syst Neurosci. 2015; 9:102. PMID: 26236203.
Article5. Ortega-Martnez S. A new perspective on the role of the CREB family of transcription factors in memory consolidation via adult hippocampal neurogenesis. Front Mol Neurosci. 2015; 8:46. PMID: 26379491.
Article6. Siegel GJ, Agranoff BW, Wayne Albers R, Fisher SK, Uhler MD. Basic Neurochemistry: Molecular, Cellular and Medical Aspects. Philadelphia: Lippincott-Raven;2005. p. 637–670.7. Simpson IA, Dwyer D, Malide D, Moley KH, Travis A, Vannucci SJ. The facilitative glucose transporter GLUT3: 20 years of distinction. Am J Physiol Endocrinol Metab. 2008; 295(2):E242–E253. PMID: 18577699.
Article8. Vannucci SJ, Maher F, Simpson IA. Glucose transporter proteins in brain: delivery of glucose to neurons and glia. Glia. 1997; 21(1):2–21. PMID: 9298843.
Article9. Guo X, Geng M, Du G. Glucose transporter 1, distribution in the brain and in neural disorders: its relationship with transport of neuroactive drugs through the blood-brain barrier. Biochem Genet. 2005; 43(3-4):175–187. PMID: 15932065.
Article10. Maher F, Vannucci SJ, Simpson IA. Glucose transporter proteins in brain. FASEB J. 1994; 8(13):1003–1011. PMID: 7926364.
Article11. Nehlig A, de Vasconcelos AP, Boyet S. Quantitative autoradiographic measurement of local cerebral glucose utilization in freely moving rats during postnatal development. J Neurosci. 1988; 8(7):2321–2333. PMID: 3249228.
Article12. Vannucci RC, Christensen MA, Stein DT. Regional cerebral glucose utilization in the immature rat: effect of hypoxiaischemia. Pediatr Res. 1989; 26(3):208–214. PMID: 2587122.
Article13. Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965; 124(3):319–335. PMID: 5861717.
Article14. Bayer SA, Altman J. Hippocampal development in the rat: cytogenesis and morphogenesis examined with autoradiography and low-level X-irradiation. J Comp Neurol. 1974; 158(1):55–79. PMID: 4430737.
Article15. Yoo DY, Yoo KY, Park JH, Choi JW, Kim W, Hwang IK, Won MH. Detailed differentiation of calbindin d-28k-immunoreactive cells in the dentate gyrus in C57BL/6 mice at early postnatal stages. Lab Anim Res. 2011; 27(2):153–159. PMID: 21826176.
Article16. Hebel R, Stromberg MW. Anatomy and Embryology of the Laboratory Rat. Wörthsee: BioMed Verlag;1986.17. Vannucci SJ. Developmental expression of GLUT1 and GLUT3 glucose transporters in rat brain. J Neurochem. 1994; 62(1):240–246. PMID: 8263524.
Article18. Aghajanian GK, Bloom FE. The formation of synaptic junctions in developing rat brain: a quantitative electron microscopic study. Brain Res. 1967; 6(4):716–727. PMID: 4169903.
Article19. Ghosh A, Greenberg ME. Calcium signaling in neurons: molecular mechanisms and cellular consequences. Science. 1995; 268(5208):239–247. PMID: 7716515.
Article20. Riccio A, Ahn S, Davenport CM, Blendy JA, Ginty DD. Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science. 1999; 286(5448):2358–2361. PMID: 10600750.
Article21. Jin N, Qian W, Yin X, Zhang L, Iqbal K, Grundke-Iqbal I, Gong CX, Liu F. CREB regulates the expression of neuronal glucose transporter 3: a possible mechanism related to impaired brain glucose uptake in Alzheimer's disease. Nucleic Acids Res. 2013; 41(5):3240–3256. PMID: 23341039.
Article22. Rajakumar A, Thamotharan S, Raychaudhuri N, Menon RK, Devaskar SU. Trans-activators regulating neuronal glucose transporter isoform-3 gene expression in mammalian neurons. J Biol Chem. 2004; 279(25):26768–26779. PMID: 15054091.
Article23. Rajakumar RA1, Thamotharan S, Menon RK, Devaskar SU. Sp1 and Sp3 regulate transcriptional activity of the facilitative glucose transporter isoform-3 gene in mammalian neuroblasts and trophoblasts. J Biol Chem. 1998; 273(42):27474–27483. PMID: 9765277.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Decrease in glucose transporter 1 levels and translocation of glucose transporter 3 in the dentate gyrus of C57BL/6 mice and gerbils with aging
- The Morphologic Changes of Parvalbumin- Immunoreactive Interneurons of the Dentate Gyrus in Kainate-Treated Mouse Hippocampal Slice Culture Epilepsy Model
- Expression Changes of c-Fos Protein of Rat Brain Following Pentylenetetrazol-induced Seizures
- Effect of acute pentylenetetrazol injection induced epileptic seizures on rat dentate gyrus at different postnatal ages
- Change of peroxisome proliferator-activated receptor gamma expression pattern in the gerbil dentate gyrus after transient global cerebral ischemia