Ann Dermatol.
2017 Apr;29(2):194-199. 10.5021/ad.2017.29.2.194.
Serial Changes of Heat Shock Protein 70 and Interleukin-8 in Burn Blister Fluid
- Affiliations
-
- 1Department of Emergency Medicine, Hangang Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea.
- 2Department of Anesthesiology and Pain Medicine, Hangang Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea. Kwak65joy@gmail.com
- 3Department of Plasticsurgery, Hangang Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea.
- 4Department of Surgery, Hangang Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea.
- 5Burn Institute, Hangang Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea.
- 6Department of Dermatology, Kangnam Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea. hyeonekim@gmail.com
- KMID: 2394843
- DOI: http://doi.org/10.5021/ad.2017.29.2.194
Abstract
- BACKGROUND
It has been reported that heat shock protein 70 (HSP70) and interleukin-8 (IL-8) play an important role in cells during the wound healing process. However, there has been no report on the effect of HSP70 and IL-8 on the blisters of burn patients.
OBJECTIVE
This study aimed to evaluate the serial quantitative changes of HSP70 and IL-8 in burn blisters.
METHODS
Twenty-five burn patients were included, for a total of 36 cases: twenty cases on the first day, six cases on the second, five cases on the third, three cases on the fourth, and two cases on the fifth. A correlation analysis was performed to determine the relationship between the concentration of HSP70 and IL-8 and the length of the treatment period.
RESULTS
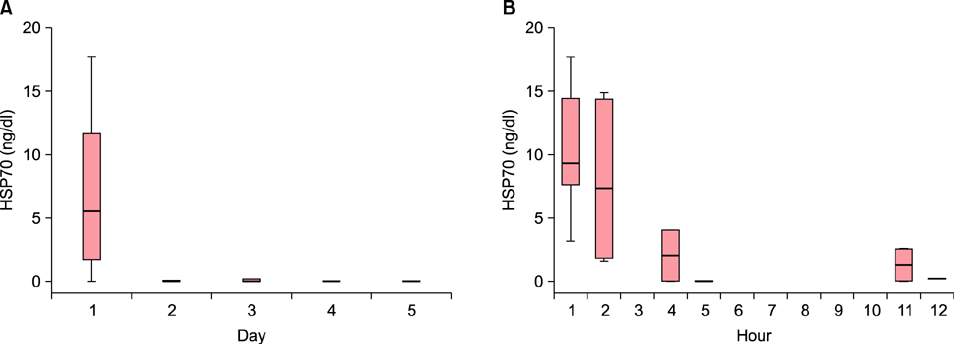
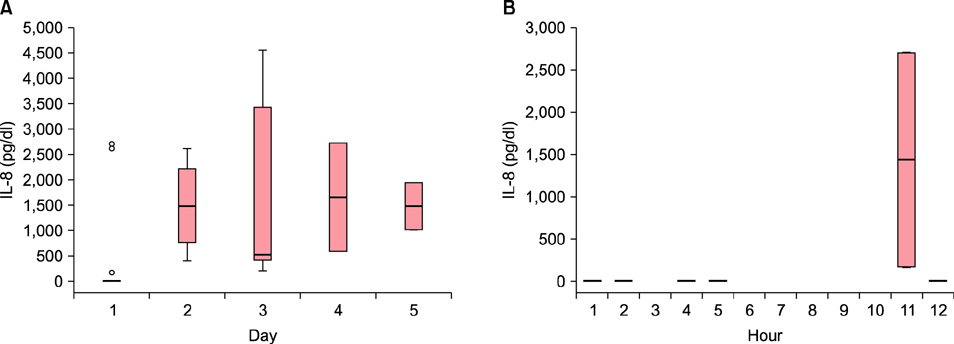
The HSP70 concentration was the highest on the first day, after which it decreased down to near zero. Most HSP70 was generated during the first 12 hours after the burn accident. There was no correlation between the concentration of HSP70 on the first day and the length of the treatment period. No measurable concentration of IL-8 was detected before 5 hours, but the concentration started to increase after 11 hours. The peak value was measured on the fourth day.
CONCLUSION
While HSP70 increased in the first few hours and decreased afterwards, IL-8 was produced after 11 hours and increased afterward in burn blister fluid. These findings provide new evidence on serial changes of inflammatory mediators in burn blister fluid.
Keyword
MeSH Terms
Figure
Reference
-
1. Pan SC, Wu LW, Chen CL, Shieh SJ, Chiu HY. Deep partial thickness burn blister fluid promotes neovascularization in the early stage of burn wound healing. Wound Repair Regen. 2010; 18:311–318.
Article2. Ono I, Gunji H, Zhang JZ, Maruyama K, Kaneko F. A study of cytokines in burn blister fluid related to wound healing. Burns. 1995; 21:352–355.
Article3. Widgerow AD, Tussardi IT, Banyard DA, King K, Chiang R, Wirth G, et al. Burn wound fluid: an important diagnostic source. Wound Healing South Afr. 2014; 7:9–12.4. Pan SC. Burn blister fluids in the neovascularization stage of burn wound healing: a comparison between superficial and deep partial-thickness burn wounds. Burns Trauma. 2013; 1:27–31.
Article5. Srivastava P. Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev Immunol. 2002; 2:185–194.
Article6. Giffard RG, Han RQ, Emery JF, Duan M, Pittet JF. Regulation of apoptotic and inflammatory cell signaling in cerebral ischemia: the complex roles of heat shock protein 70. Anesthesiology. 2008; 109:339–348.
Article7. Henderson B. Integrating the cell stress response: a new view of molecular chaperones as immunological and physiological homeostatic regulators. Cell Biochem Funct. 2010; 28:1–14.
Article8. Kennedy D, Mnich K, Samali A. Heat shock preconditioning protects against ER stress-induced apoptosis through the regulation of the BH3-only protein BIM. FEBS Open Bio. 2014; 4:813–821.
Article9. Sajjadi AY, Mitra K, Grace M. Expression of heat shock proteins 70 and 47 in tissues following short-pulse laser irradiation: assessment of thermal damage and healing. Med Eng Phys. 2013; 35:1406–1414.
Article10. Kim S, Kwon J. Thymosin β4 has a major role in dermal burn wound healing that involves actin cytoskeletal remodelling via heat-shock protein 70. J Tissue Eng Regen Med. 2015; DOI: 10.1002/term.2028. [Epub ahead of print].11. Xiong L, Sun J, Hirche C, Yang J, Yang Y, Xia Y, et al. In vitro N-acetyl-L-cysteine promotes proliferation and suppresses interleukin-8 expression in adipose-derived stem cells. Aesthetic Plast Surg. 2012; 36:1260–1265.
Article12. Ashcroft GS, Mills SJ, Lei K, Gibbons L, Jeong MJ, Taniguchi M, et al. Estrogen modulates cutaneous wound healing by downregulating macrophage migration inhibitory factor. J Clin Invest. 2003; 111:1309–1318.
Article13. Nwariaku F, Sikes PJ, Lightfoot E Jr, Mileski WJ. Inhibition of selectin- and integrin-mediated inflammatory response after burn injury. J Surg Res. 1996; 63:355–358.
Article14. Iocono JA, Colleran KR, Remick DG, Gillespie BW, Ehrlich HP, Garner WL. Interleukin-8 levels and activity in delayed-healing human thermal wounds. Wound Repair Regen. 2000; 8:216–225.
Article15. Fujita T, Yoshimoto T, Matsuda S, Kajiya M, Kittaka M, Imai H, et al. Interleukin-8 induces DNA synthesis, migration and down-regulation of cleaved caspase-3 in cultured human gingival epithelial cells. J Periodontal Res. 2015; 50:479–485.
Article16. Michel G, Kemény L, Peter RU, Beetz A, Ried C, Arenberger P, et al. Interleukin-8 receptor-mediated chemotaxis of normal human epidermal cells. FEBS Lett. 1992; 305:241–243.
Article17. Rennekampff HO, Hansbrough JF, Kiessig V, Doré C, Sticherling M, Schröder JM. Bioactive interleukin-8 is expressed in wounds and enhances wound healing. J Surg Res. 2000; 93:41–54.
Article18. Sargent RL. Management of blisters in the partial-thickness burn: an integrative research review. J Burn Care Res. 2006; 27:66–81.
Article19. Cleland H. Thermal burns--assessment and acute management in the general practice setting. Aust Fam Physician. 2012; 41:372–375.20. duKamp A. Deroofing minor burn blisters--what is the evidence? Accid Emerg Nurs. 2001; 9:217–221.
Article21. Ireland HE, Leoni F, Altaie O, Birch CS, Coleman RC, Hunter-Lavin C, et al. Measuring the secretion of heat shock proteins from cells. Methods. 2007; 43:176–183.
Article22. Kim JY, Yenari MA. The immune modulating properties of the heat shock proteins after brain injury. Anat Cell Biol. 2013; 46:1–7.
Article23. Weissman C. The metabolic response to stress: an overview and update. Anesthesiology. 1990; 73:308–327.24. Ono SJ, Nakamura T, Miyazaki D, Ohbayashi M, Dawson M, Toda M. Chemokines: roles in leukocyte development, trafficking, and effector function. J Allergy Clin Immunol. 2003; 111:1185–1199. quiz 1200.
Article25. Benkoe T, Baumann S, Weninger M, Pones M, Reck C, Rebhandl W, et al. Comprehensive evaluation of 11 cytokines in premature infants with surgical necrotizing enterocolitis. PLoS One. 2013; 8:e58720.
Article26. Chan KY, Leung FW, Lam HS, Tam YH, To KF, Cheung HM, et al. Immunoregulatory protein profiles of necrotizing enterocolitis versus spontaneous intestinal perforation in preterm infants. PLoS One. 2012; 7:e36977.
Article27. Berney T, Gasche Y, Robert J, Jenny A, Mensi N, Grau G, et al. Serum profiles of interleukin-6, interleukin-8, and interleukin-10 in patients with severe and mild acute pancreatitis. Pancreas. 1999; 18:371–377.
Article28. Lee YB, Kim SJ, Park SM, Lee KH, Han HJ, Yu DS, et al. Immunomodulatory effects of Deokgu thermomineral water balneotherapy on oxazolone-induced atopic dermatitis murine model. Ann Dermatol. 2016; 28:192–198.
Article29. Luo X, Zuo X, Zhou Y, Zhang B, Shi Y, Liu M, et al. Extracellular heat shock protein 70 inhibits tumour necrosis factor-alpha induced proinflammatory mediator production in fibroblast-like synoviocytes. Arthritis Res Ther. 2008; 10:R41.30. Krause M, Heck TG, Bittencourt A, Scomazzon SP, Newsholme P, Curi R, et al. The chaperone balance hypothesis: the importance of the extracellular to intracellular HSP70 ratio to inflammation-driven type 2 diabetes, the effect of exercise, and the implications for clinical management. Mediators Inflamm. 2015; 2015:249205.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Heat Shock Protein 70 m-RNA in Rat Bladder Overdistended by Diuresis
- Environmental factors regulating the expression of Porphyromonas gingivalis heat shock protein
- The Protective Effect of Induced Heat Shock Protein in Human Corneal Epithelial Cells
- Heat Shock Protein 90 (HSP90) and Immune Regulation
- Heat Shock Protein Induction By An Infrared Warm Compression Device