Ann Dermatol.
2017 Apr;29(2):149-155. 10.5021/ad.2017.29.2.149.
Low-Level Light Therapy with 410 nm Light Emitting Diode Suppresses Collagen Synthesis in Human Keloid Fibroblasts: An In Vitro Study
- Affiliations
-
- 1Department of Dermatology, Ajou University School of Medicine, Suwon, Korea. maychan@ajou.ac.kr
- 2Department of Biochemistry, Ajou University School of Medicine, Suwon, Korea.
- 3Laboratory of Cell Biology, Ajou University School of Medicine, Suwon, Korea.
- KMID: 2394837
- DOI: http://doi.org/10.5021/ad.2017.29.2.149
Abstract
- BACKGROUND
Keloids are characterized by excessive collagen deposition in the dermis, in which transforming growth factor β (TGF-β)/Smad signaling plays an important role. Low-level light therapy (LLLT) is reported as effective in preventing keloids in clinical reports, recently. To date, studies investigating the effect of LLLT on keloid fibroblasts are extremely rare.
OBJECTIVE
We investigated the effect of LLLT with blue (410 nm), red (630 nm), and infrared (830 nm) light on the collagen synthesis in keloid fibroblasts.
METHODS
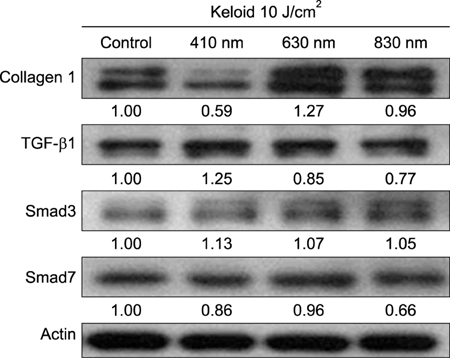
Keloid fibroblasts were isolated from keloid-revision surgery samples and irradiated using 410-, 630-, 830-nm light emitting diode twice, with a 24-hour interval at 10 J/cm². After irradiation, cells were incubated for 24 and 48 hours and real-time quantitative reverse transcription polymerase chain reaction was performed. Western blot analysis was also performed in 48 hours after last irradiation. The genes and proteins of collagen type I, TGF-β1, Smad3, and Smad7 were analyzed.
RESULTS
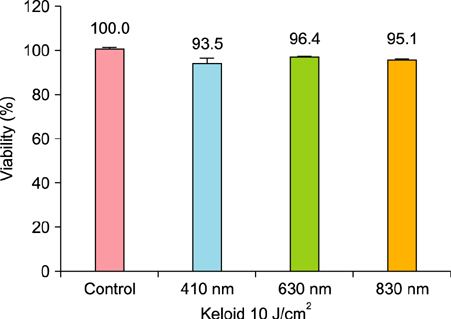
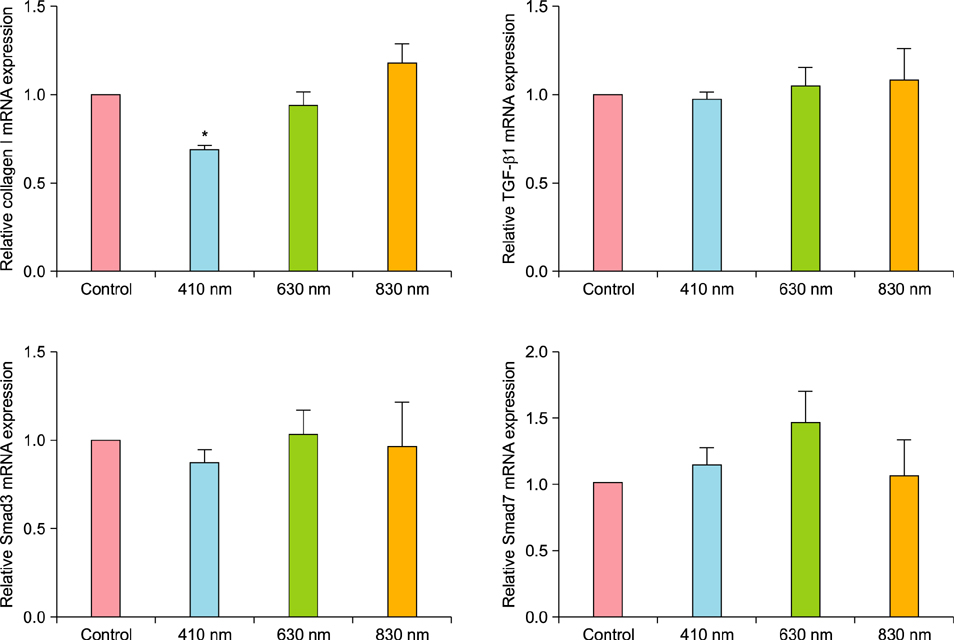
We observed no statistically significant change in the viability of keloid fibroblasts after irradiation. Collagen type I was the only gene whose expression significantly decreased after irradiation at 410 nm when compared to the non-irradiated control. Western blot analysis showed that LLLT at 410 nm lowered the protein levels of collagen type I compared to the control.
CONCLUSION
LLLT at 410 nm decreased the expression of collagen type I in keloid fibroblasts and might be effective in preventing keloid formation in their initial stage.
MeSH Terms
Figure
Reference
-
1. Tsujita-Kyutoku M, Uehara N, Matsuoka Y, Kyutoku S, Ogawa Y, Tsubura A. Comparison of transforming growth factor-beta/Smad signaling between normal dermal fibroblasts and fibroblasts derived from central and peripheral areas of keloid lesions. In Vivo. 2005; 19:959–963.2. Park SY, Park JY, Kim CH, Kang SU, Kim JH, Bark KM, et al. Effects of Xanthium stramarium and Psoralea corylifolia extracts combined with UVA1 irradiation on the cell proliferation and TGF-β1 expression of keloid fibroblasts. Ann Dermatol. 2013; 25:304–309.
Article3. Chin GS, Liu W, Peled Z, Lee TY, Steinbrech DS, Hsu M, et al. Differential expression of transforming growth factor-beta receptors I and II and activation of Smad 3 in keloid fibroblasts. Plast Reconstr Surg. 2001; 108:423–429.
Article4. Jagadeesan J, Bayat A. Transforming growth factor beta (TGFbeta) and keloid disease. Int J Surg. 2007; 5:278–285.5. Phan TT, Lim IJ, Aalami O, Lorget F, Khoo A, Tan EK, et al. Smad3 signalling plays an important role in keloid pathogenesis via epithelial-mesenchymal interactions. J Pathol. 2005; 207:232–242.
Article6. Mamalis AD, Lev-Tov H, Nguyen DH, Jagdeo JR. Laser and light-based treatment of Keloids--a review. J Eur Acad Dermatol Venereol. 2014; 28:689–699.7. Barolet D, Boucher A. Prophylactic low-level light therapy for the treatment of hypertrophic scars and keloids: a case series. Lasers Surg Med. 2010; 42:597–601.
Article8. Carvalho RL, Alcântara PS, Kamamoto F, Cressoni MD, Casarotto RA. Effects of low-level laser therapy on pain and scar formation after inguinal herniation surgery: a randomized controlled single-blind study. Photomed Laser Surg. 2010; 28:417–422.
Article9. da Silva JP, da Silva MA, Almeida AP, Lombardi Junior I, Matos AP. Laser therapy in the tissue repair process: a literature review. Photomed Laser Surg. 2010; 28:17–21.
Article10. Hamblin MR, Demidova T. Mechanisms of low level light therapy. Proc of SPIE. 2006; 6140:614001–614012.
Article11. Mamalis A, Garcha M, Jagdeo J. Light emitting diode-generated blue light modulates fibrosis characteristics: fibroblast proliferation, migration speed, and reactive oxygen species generation. Lasers Surg Med. 2015; 47:210–215.
Article12. Gorgidze LA, Oshemkova SA, Vorobjev IA. Blue light inhibits mitosis in tissue culture cells. Biosci Rep. 1998; 18:215–224.
Article13. Eichler M, Lavi R, Shainberg A, Lubart R. Flavins are source of visible-light-induced free radical formation in cells. Lasers Surg Med. 2005; 37:314–319.
Article14. Opländer C, Hidding S, Werners FB, Born M, Pallua N, Suschek CV. Effects of blue light irradiation on human dermal fibroblasts. J Photochem Photobiol B. 2011; 103:118–125.
Article15. Bonatti S, Hochman B, Tucci-Viegas VM, Furtado F, Pinfildi CE, Pedro AC, et al. In vitro effect of 470 nm LED (Light Emitting Diode) in keloid fibroblasts. Acta Cir Bras. 2011; 26:25–30.
Article16. Seo YK, Park JK, Song C, Kwon SY. Comparison of light-emitting diode wavelength on activity and migration of rabbit ACL cells. Lasers Med Sci. 2014; 29:245–255.
Article17. He S, Liu X, Yang Y, Huang W, Xu S, Yang S, et al. Mechanisms of transforming growth factor beta(1)/Smad signalling mediated by mitogen-activated protein kinase pathways in keloid fibroblasts. Br J Dermatol. 2010; 162:538–546.
Article18. Lim IJ, Phan TT, Tan EK, Nguyen TT, Tran E, Longaker MT, et al. Synchronous activation of ERK and phosphatidylinositol 3-kinase pathways is required for collagen and extracellular matrix production in keloids. J Biol Chem. 2003; 278:40851–40858.
Article19. Kuo YR, Wu WS, Jeng SF, Huang HC, Yang KD, Sacks JM, et al. Activation of ERK and p38 kinase mediated keloid fibroblast apoptosis after flashlamp pulsed-dye laser treatment. Lasers Surg Med. 2005; 36:31–37.
Article20. Kuo YR, Wu WS, Wang FS. Flashlamp pulsed-dye laser suppressed TGF-beta1 expression and proliferation in cultured keloid fibroblasts is mediated by MAPK pathway. Lasers Surg Med. 2007; 39:358–364.
Article21. Igota S, Tosa M, Murakami M, Egawa S, Shimizu H, Hyakusoku H, et al. Identification and characterization of Wnt signaling pathway in keloid pathogenesis. Int J Med Sci. 2013; 10:344–354.
Article22. Babu M, Diegelmann R, Oliver N. Keloid fibroblasts exhibit an altered response to TGF-beta. J Invest Dermatol. 1992; 99:650–655.23. Younai S, Nichter LS, Wellisz T, Reinisch J, Nimni ME, Tuan TL. Modulation of collagen synthesis by transforming growth factor-beta in keloid and hypertrophic scar fibroblasts. Ann Plast Surg. 1994; 33:148–151.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Low Level Light Therapy Using an 830-nm Light Emitting Diode Promotes Wound Healing via TGF-β/SMAD Pathway Activation
- The shear bond strength and adhesive failure pattern in bracket bonding with different light-curing methods
- Cutaneous Photorejuvenation of Light Emitting Diodes via the Melatonin Membrane Receptor Pathway
- Effects of 630 nm Light-Emitting Diode Irradiation on Caveolin-1 and Procollagen I and III Expression in Human Dermal Fibroblasts
- Topical Photodynamic Therapy for Treatment of Actinic Keratosis Using Light-Emitting Diode (LED) Device