Cancer Res Treat.
2017 Oct;49(4):1001-1011. 10.4143/crt.2016.546.
Randomized Phase II Study of Afatinib Plus Simvastatin Versus Afatinib Alone in Previously Treated Patients with Advanced Nonadenocarcinomatous Non-small Cell Lung Cancer
- Affiliations
-
- 1Center for Lung Cancer, Research Institute and Hospital, National Cancer Center, Goyang, Korea. jymama@ncc.re.kr
- 2Department of Hematology-Oncology, Chungbuk National University Hospital, Cheongju, Korea.
- 3Biometric Research Branch, Research Institute and Hospital, National Cancer Center, Goyang, Korea.
- KMID: 2394819
- DOI: http://doi.org/10.4143/crt.2016.546
Abstract
- PURPOSE
This phase II study examined whether the addition of simvastatin to afatinib provides a clinical benefit compared with afatinib monotherapy in previously treated patients with nonadenocarcinomatous non-small cell lung cancer (NA-NSCLC).
MATERIALS AND METHODS
Patients with advanced NA-NSCLC who progressed after one or two chemotherapy regimens were randomly assigned to a simvastatin (40 mg/day) plus afatinib (40 mg/day) (AS) arm or to an afatinib (A) arm. The primary endpoint was response rate (RR).
RESULTS
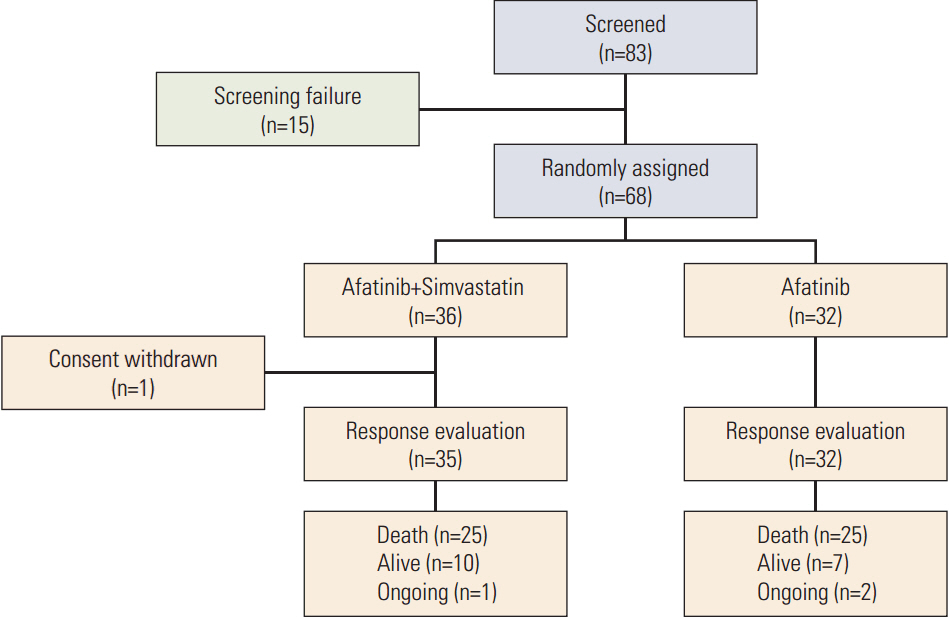
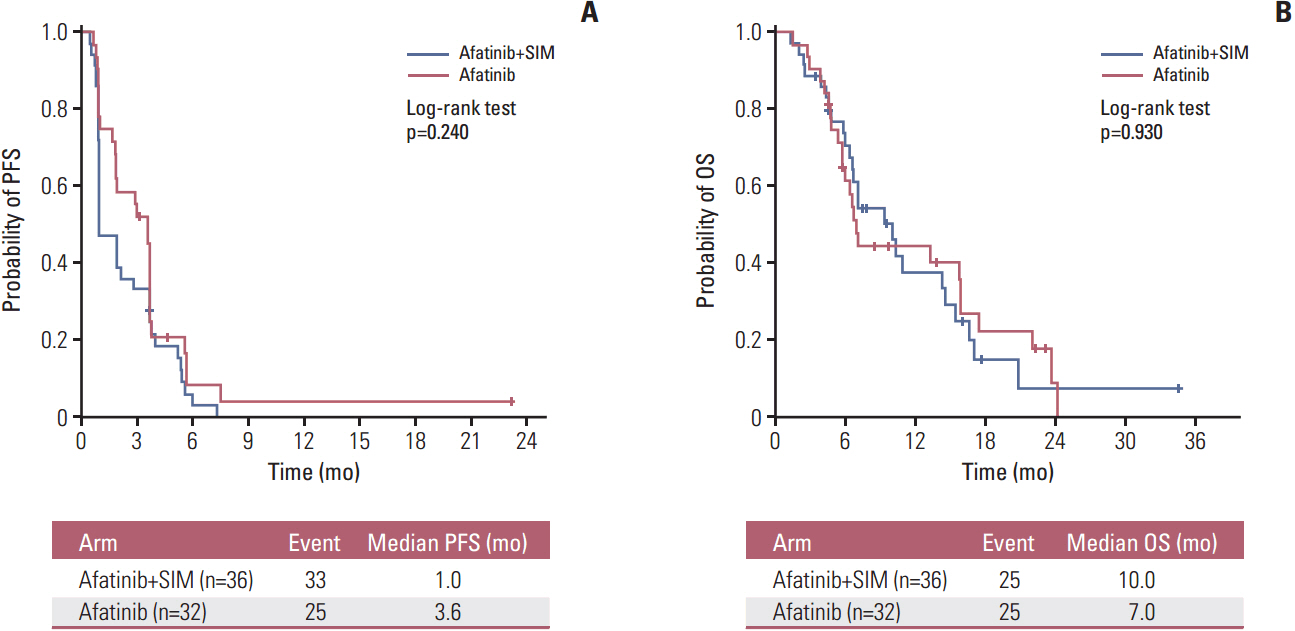
Sixty-eight patients were enrolled (36 in the AS arm and 32 in the A arm). The RR was 5.7% (95% confidence interval [CI], 0.7 to 19.2) for AS and 9.4% (95% CI, 2.0 to 25.0) for A (p=0.440). In arms AS and A, the median progression-free survival (PFS) was 1.0 versus 3.6 months (p=0.240) and the overall survival was 10.0 months versus 7.0 months (p=0.930), respectively. Skin rash, stomatitis, and diarrhea were the most common adverse events in both arms. More grade 3 or 4 diarrhea was observed in arm A (18.8% vs. 5.6% in arm AS). In all patients, the median PFS for treatment including afatinib was not correlated with the status of epidermal growth factor receptor (EGFR) mutation (p=0.122), EGFR fluorescence in situ hybridization (p=0.944), or EGFR immunohistochemistry (p=0.976). However, skin rash severity was significantly related to the risk of progression for afatinib (hazard ratio for skin rash grade ≥ 2 vs. grade < 2, 0.44; 95% CI, 0.25 to 0.78; p=0.005).
CONCLUSION
There were no significant differences in the efficacy between AS and A arms in patients with NA-NSCLC.
Keyword
MeSH Terms
-
Arm
Carcinoma, Non-Small-Cell Lung*
Carcinoma, Squamous Cell
Diarrhea
Disease-Free Survival
Drug Therapy
Exanthema
Fluorescence
Humans
Hydroxymethylglutaryl-CoA Reductase Inhibitors
Immunohistochemistry
In Situ Hybridization
Receptor, Epidermal Growth Factor
Simvastatin*
Stomatitis
Receptor, Epidermal Growth Factor
Simvastatin
Figure
Reference
-
References
1. Gazdar AF. Epidermal growth factor receptor inhibition in lung cancer: the evolving role of individualized therapy. Cancer Metastasis Rev. 2010; 29:37–48.
Article2. Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of nonsmall-cell lung cancer to gefitinib. N Engl J Med. 2004; 350:2129–39.
Article3. Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004; 304:1497–500.4. Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010; 11:121–8.
Article5. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010; 362:2380–8.
Article6. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as firstline treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012; 13:239–46.7. Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-smallcell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011; 12:735–42.
Article8. Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang Y, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014; 15:213–22.
Article9. Lee JK, Hahn S, Kim DW, Suh KJ, Keam B, Kim TM, et al. Epidermal growth factor receptor tyrosine kinase inhibitors vs conventional chemotherapy in non-small cell lung cancer harboring wild-type epidermal growth factor receptor: a metaanalysis. JAMA. 2014; 311:1430–7.10. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated nonsmall-cell lung cancer. N Engl J Med. 2005; 353:123–32.
Article11. Han JY, Lee SH, Yoo NJ, Hyung LS, Moon YJ, Yun T, et al. A randomized phase II study of gefitinib plus simvastatin versus gefitinib alone in previously treated patients with advanced non-small cell lung cancer. Clin Cancer Res. 2011; 17:1553–60.
Article12. Cortot AB, Janne PA. Molecular mechanisms of resistance in epidermal growth factor receptor-mutant lung adenocarcinomas. Eur Respir Rev. 2014; 23:356–66.
Article13. Endo A. The discovery and development of HMG-CoA reductase inhibitors. 1992. Atheroscler Suppl. 2004; 5:67–80.14. Seabra MC, Mules EH, Hume AN. Rab GTPases, intracellular traffic and disease. Trends Mol Med. 2002; 8:23–30.
Article15. Pruitt K, Der CJ. Ras and Rho regulation of the cell cycle and oncogenesis. Cancer Lett. 2001; 171:1–10.
Article16. Mantha AJ, Hanson JE, Goss G, Lagarde AE, Lorimer IA, Dimitroulakos J. Targeting the mevalonate pathway inhibits the function of the epidermal growth factor receptor. Clin Cancer Res. 2005; 11:2398–407.
Article17. Park IH, Kim JY, Jung JI, Han JY. Lovastatin overcomes gefitinib resistance in human non-small cell lung cancer cells with K-Ras mutations. Invest New Drugs. 2010; 28:791–9.
Article18. Prueksaritanont T, Ma B, Yu N. The human hepatic metabolism of simvastatin hydroxy acid is mediated primarily by CYP3A, and not CYP2D6. Br J Clin Pharmacol. 2003; 56:120–4.
Article19. Peters S, Zimmermann S, Adjei AA. Oral epidermal growth factor receptor tyrosine kinase inhibitors for the treatment of non-small cell lung cancer: comparative pharmacokinetics and drug-drug interactions. Cancer Treat Rev. 2014; 40:917–26.
Article20. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–47.
Article21. Lee DH, Lee GK, Kong SY, Kook MC, Yang SK, Park SY, et al. Epidermal growth factor receptor status in anaplastic thyroid carcinoma. J Clin Pathol. 2007; 60:881–4.
Article22. Hirsch FR, Varella-Garcia M, Bunn PA Jr, Di Maria MV, Veve R, Bremmes RM, et al. Epidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. J Clin Oncol. 2003; 21:3798–807.
Article23. Soria JC, Felip E, Cobo M, Lu S, Syrigos K, Lee KH, et al. Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2015; 16:897–907.
Article24. Kudo K, Hotta K, Bessho A, Nogami N, Kozuki T, Kuyama S, et al. Development of a skin rash within the first week and the therapeutic effect in afatinib monotherapy for EGFR-mutant non-small cell lung cancer (NSCLC): Okayama Lung Cancer Study Group experience. Cancer Chemother Pharmacol. 2016; 77:1005–9.
Article25. Lee Y, Shim HS, Park MS, Kim JH, Ha SJ, Kim SH, et al. High EGFR gene copy number and skin rash as predictive markers for EGFR tyrosine kinase inhibitors in patients with advanced squamous cell lung carcinoma. Clin Cancer Res. 2012; 18:1760–8.
Article26. Hata A, Katakami N, Kunimasa K, Yoshioka H, Fujita S, Kaji R, et al. Erlotinib for pretreated squamous cell carcinoma of the lung in Japanese patients. Jpn J Clin Oncol. 2011; 41:1366–72.
Article27. Cappuzzo F, Hirsch FR, Rossi E, Bartolini S, Ceresoli GL, Bemis L, et al. Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small-cell lung cancer. J Natl Cancer Inst. 2005; 97:643–55.
Article28. Wong WW, Dimitroulakos J, Minden MD, Penn LZ. HMGCoA reductase inhibitors and the malignant cell: the statin family of drugs as triggers of tumor-specific apoptosis. Leukemia. 2002; 16:508–19.
Article29. Demierre MF, Higgins PD, Gruber SB, Hawk E, Lippman SM. Statins and cancer prevention. Nat Rev Cancer. 2005; 5:930–42.
Article30. Lee J, Hong YS, Hong JY, Han SW, Kim TW, Kang HJ, et al. Effect of simvastatin plus cetuximab/irinotecan for KRAS mutant colorectal cancer and predictive value of the RAS signature for treatment response to cetuximab. Invest New Drugs. 2014; 32:535–41.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Afatinib for Lung Cancer on Papillary Thyroid Carcinoma
- Efficacy and Safety of Afatinib for EGFR-mutant Non-small Cell Lung Cancer, Compared with Gefitinib or Erlotinib
- Efficacy of Afatinib in a Previously-Treated Patient with Non-Small Cell Lung Cancer Harboring HER2 Mutation: Case Report
- The Role of Brain Radiotherapy before First-Line Afatinib Therapy, Compared to Gefitinib or Erlotinib, in Patients with EGFR-Mutant Non–Small Cell Lung Cancer
- Afatinib Mediates Autophagic Degradation of ORAI1, STIM1, and SERCA2, Which Inhibits Proliferation of Non–Small Cell Lung Cancer Cells