J Korean Assoc Oral Maxillofac Surg.
2017 Oct;43(5):324-330. 10.5125/jkaoms.2017.43.5.324.
Immunohistochemical evaluation of p63 and cyclin D1 in oral squamous cell carcinoma and leukoplakia
- Affiliations
-
- 1Department of Oral Pathology, Ahmedabad Dental College, Ahmedabad, India.
- 2Department of Oral Biology, Basic Dental Sciences, Faculty of Dentistry, Al-Huwaiyah, Taif University, Taif, Kingdom of Saudi Arabia. drmanju26@hotmail.com
- 3Department of Oral Pathology, K.M.Shah Dental College, Vadodara, India.
- 4Department of Oral Pathology, Karnavati School of Dentistry, Gandhinagar, India.
- 5Department of Oral Pathology, Vaidik Dental College, Daman, India.
- KMID: 2393598
- DOI: http://doi.org/10.5125/jkaoms.2017.43.5.324
Abstract
OBJECTIVES
There are only a limited number of studies on cyclin D1 and p63 expression in oral squamous cell carcinoma (OSCC) and leukoplakia. This study compared cyclin D1 and p63 expression in leukoplakia and OSCC to investigate the possible correlation of both markers with grade of dysplasia and histological grade of OSCC.
MATERIALS AND METHODS
The study included a total of 60 cases, of which 30 were diagnosed with OSCC and 30 with leukoplakia, that were evaluated immunohistochemically for p63 and cyclin D1 expression. Protein expression was correlated based on grades of dysplasia and OSCC.
RESULTS
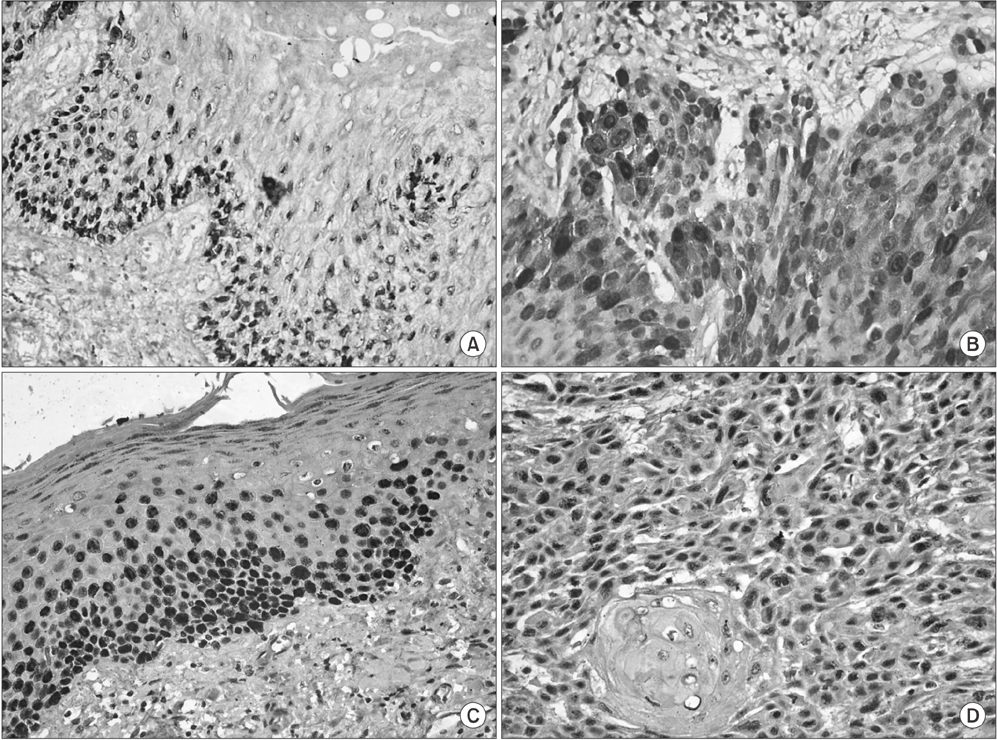
Out of 30 cases of OSCC, 23 cases (76.7%) were cyclin D1 positive and 30 cases (100%) were p63 positive. Out of 30 cases of leukoplakia, 21 cases (70.0%) were cyclin D1 positive and 30 (100%) were p63 positive (P<0.05).
CONCLUSION
The overall expression of cyclin D1 and p63 correlated with tumor differentiation, and increases were correlated with poor histological grades, from well-differentiated to poorly-differentiated SCC. Increased cyclin D1 and p63 expression was associated with the severity of leukoplakia. Based on these results cyclin D1 and p63 products can be a useful tool for improved leukoplakia prognosis.
MeSH Terms
Figure
Cited by 1 articles
-
Role of E-cadherin and cyclin D1 as predictive markers of aggression and clonal expansion in head and neck squamous cell carcinoma
Khushdeep Shergill, Arijit Sen, Hari Janardanan Pillai
J Korean Assoc Oral Maxillofac Surg. 2018;44(4):182-190. doi: 10.5125/jkaoms.2018.44.4.182.
Reference
-
1. Fregonesi PA, Teresa DB, Duarte RA, Neto CB, de Oliveira MR, Soares CP. p16(INK4A) immunohistochemical overexpression in premalignant and malignant oral lesions infected with human papillomavirus. J Histochem Cytochem. 2003; 51:1291–1297.2. Peghini PE, Fehr J. Analysis of cyclin D1 expression by quantitative real-time reverse transcription-polymerase chain reaction in the diagnosis of mantle cell lymphoma. Am J Clin Pathol. 2002; 117:237–245.
Article3. Todd R, Hinds PW, Munger K, Rustgi AK, Opitz OG, Suliman Y, et al. Cell cycle dysregulation in oral cancer. Crit Rev Oral Biol Med. 2002; 13:51–61.4. Hosokawa Y, Arnold A. Cyclin D1/PRAD1 as a central target in oncogenesis. J Lab Clin Med. 1996; 127:246–252.
Article5. Fracchiolla NS, Pruneri G, Pignataro L, Carboni N, Capaccio P, Boletini A, et al. Molecular and immunohistochemical analysis of the bcl-1/cyclin D1 gene in laryngeal squamous cell carcinomas: correlation of protein expression with lymph node metastases and advanced clinical stage. Cancer. 1997; 79:1114–1121.
Article6. Hu H, Xia SH, Li AD, Xu X, Cai Y, Han YL, et al. Elevated expression of p63 protein in human esophageal squamous cell carcinomas. Int J Cancer. 2002; 102:580–583.
Article7. Shah RB, Zhou M, LeBlanc M, Snyder M, Rubin MA. Comparison of the basal cell-specific markers, 34betaE12 and p63, in the diagnosis of prostate cancer. Am J Surg Pathol. 2002; 26:1161–1168.
Article8. Castle JT, Cardinali M, Kratochvil FJ, Abbondanzo SL, Kessler HP, Auclair PL, et al. P53 and cyclin D1 staining patterns of malignant and premalignant oral lesions in age-dependent populations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999; 88:326–332.
Article9. Poon RYC. Cell cycle control. Encyclopedia Cancer. 1997; 1:246–255.
Article10. Kumar V, Cotran RS, Robbins SL. Robbins basic pathology. 7th ed. Philadelphia: Saunders;2003.11. Weinstein IB, Zhou P. Cell cycle control gene defects and human cancer. Encyclopedia Cancer. 1997; 1:256–267.12. Mate JL, Ariza A, Aracil C, López D, Isamat M, Pérez-Piteira J, et al. Cyclin D1 overexpression in non-small cell lung carcinoma: correlation with Ki67 labelling index and poor cytoplasmic differentiation. J Pathol. 1996; 180:395–399.
Article13. Angadi PV, Krishnapillai R. Cyclin D1 expression in oral squamous cell carcinoma and verrucous carcinoma: correlation with histological differentiation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007; 103:e30–e35.
Article14. Lam KY, Ng IO, Yuen AP, Kwong DL, Wei W. Cyclin D1 expression in oral squamous cell carcinomas: clinicopathological relevance and correlation with p53 expression. J Oral Pathol Med. 2000; 29:167–172.
Article15. Goto H, Kawano K, Kobayashi I, Sakai H, Yanagisawa S. Expression of cyclin D1 and GSK-3beta and their predictive value of prognosis in squamous cell carcinomas of the tongue. Oral Oncol. 2002; 38:549–556.
Article16. Bartkova J, Lukas J, Strauss M, Bartek J. Cell cycle-related variation and tissue-restricted expression of human cyclin D1 protein. J Pathol. 1994; 172:237–245.
Article17. Zhang YJ, Jiang W, Chen CJ, Lee CS, Kahn SM, Santella RM, et al. Amplification and overexpression of cyclin D1 in human hepatocellular carcinoma. Biochem Biophys Res Commun. 1993; 196:1010–1016.
Article18. Ruhul Quddus M, Latkovich P, Castellani WJ, James Sung C, Steinhoff MM, Briggs RC, et al. Expression of cyclin D1 in normal, metaplastic, hyperplastic endometrium and endometrioid carcinoma suggests a role in endometrial carcinogenesis. Arch Pathol Lab Med. 2002; 126:459–463.
Article19. Tsuruta H, Sakamoto H, Onda M, Terada M. Amplification and overexpression of EXP1 and EXP2/Cyclin D1 genes in human esophageal carcinomas. Biochem Biophys Res Commun. 1993; 196:1529–1536.
Article20. Naitoh H, Shibata J, Kawaguchi A, Kodama M, Hattori T. Overexpression and localization of cyclin D1 mRNA and antigen in esophageal cancer. Am J Pathol. 1995; 146:1161–1169.21. Mills AA. p63: oncogene or tumor suppressor. Curr Opin Genet Dev. 2006; 16:38–44.
Article22. Foschini MP, Gaiba A, Cocchi R, Pennesi MG, Gatto MR, Frezza GP, et al. Pattern of p63 expression in squamous cell carcinoma of the oral cavity. Virchows Arch. 2004; 444:332–339.
Article23. Bortoluzzi MC, Yurgel LS, Dekker NP, Jordan RC, Regezi JA. Assessment of p63 expression in oral squamous cell carcinomas and dysplasias. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004; 98:698–704.
Article24. Chen YK, Hsue SS, Lin LM. Expression of p63 protein and mRNA in oral epithelial dysplasia. J Oral Pathol Med. 2005; 34:232–239.
Article25. de Oliveira LR, Ribeiro-Silva A, Zucoloto S. Prognostic impact of p53 and p63 immunoexpression in oral squamous cell carcinoma. J Oral Pathol Med. 2007; 36:191–197.
Article26. Saintigny P, El-Naggar AK, Papadimitrakopoulou V, Ren H, Fan YH, Feng L, et al. DeltaNp63 overexpression, alone and in combination with other biomarkers, predicts the development of oral cancer in patients with leukoplakia. Clin Cancer Res. 2009; 15:6284–6291.
Article27. Takeda T, Sugihara K, Hirayama Y, Hirano M, Tanuma JI, Semba I. Immunohistological evaluation of Ki-67, p63, CK19 and p53 expression in oral epithelial dysplasias. J Oral Pathol Med. 2006; 35:369–375.
Article28. Vered M, Allon I, Dayan D. Maspin, p53, p63, and Ki-67 in epithelial lesions of the tongue: from hyperplasia through dysplasia to carcinoma. J Oral Pathol Med. 2009; 38:314–320.
Article29. Choi HR, Batsakis JG, Zhan F, Sturgis E, Luna MA, El-Naggar AK. Differential expression of p53 gene family members p63 and p73 in head and neck squamous tumorigenesis. Hum Pathol. 2002; 33:158–164.
Article30. Sakiz D, Turkmenoglu TT, Kabukcuoglu F. The expression of p63 and p53 in keratoacanthoma and intraepidermal and invasive neoplasms of the skin. Pathol Res Pract. 2009; 205:589–594.
Article31. Moergel M, Abt E, Stockinger M, Kunkel M. Overexpression of p63 is associated with radiation resistance and prognosis in oral squamous cell carcinoma. Oral Oncol. 2010; 46:667–671.
Article32. Chen YK, Hsue SS, Lin LM. Immunohistochemical demonstration of p63 in DMBA-induced hamster buccal pouch squamous cell carcinogenesis. Oral Dis. 2003; 9:235–240.
Article33. Faridoni-Laurens L, Bosq J, Janot F, Vayssade M, Le Bihan ML, Kaghad M, et al. P73 expression in basal layers of head and neck squamous epithelium: a role in differentiation and carcinogenesis in concert with p53 and p63. Oncogene. 2001; 20:5302–5312.
Article34. Shirendeb U, Hishikawa Y, Moriyama S, Win N, Thu MM, Mar KS, et al. Human papillomavirus infection and its possible correlation with p63 expression in cervical cancer in Japan, Mongolia, and Myanmar. Acta Histochem Cytochem. 2009; 42:181–190.
Article35. Di Como CJ, Urist MJ, Babayan I, Drobnjak M, Hedvat CV, Teruya-Feldstein J, et al. p63 expression profiles in human normal and tumor tissues. Clin Cancer Res. 2002; 8:494–501.36. Edwards PC, Bhuiya T, Kelsch RD. Assessment of p63 expression in the salivary gland neoplasms adenoid cystic carcinoma, polymorphous low-grade adenocarcinoma, and basal cell and canalicular adenomas. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004; 97:613–619.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Amplification and Overexpression of Cyclin D1 in Head and Neck Squamous Cell Carcinomas
- Expression of p16 Protein and Cyclin D1 Protein in Head and Neck Squamous Cell Carcinomas
- A case presentation of verrucous carcinoma originated from verrrucous lekoplakia
- The immunohistochemical study on the expression of p53 protein and cyclin D1 in oral squamous cell carcinomas
- A Case of Squamous Cell Carcinoma Arising from Leukoplakia