Immune Netw.
2014 Oct;14(5):260-264. 10.4110/in.2014.14.5.260.
Immunomodulatory Effects of ZYM-201 on LPS-stimulated B Cells
- Affiliations
-
- 1Department of Bioinformatics and Life Science, Soongsil University, Seoul 156-743, Korea. kimmy@ssu.ac.kr
- KMID: 2391723
- DOI: http://doi.org/10.4110/in.2014.14.5.260
Abstract
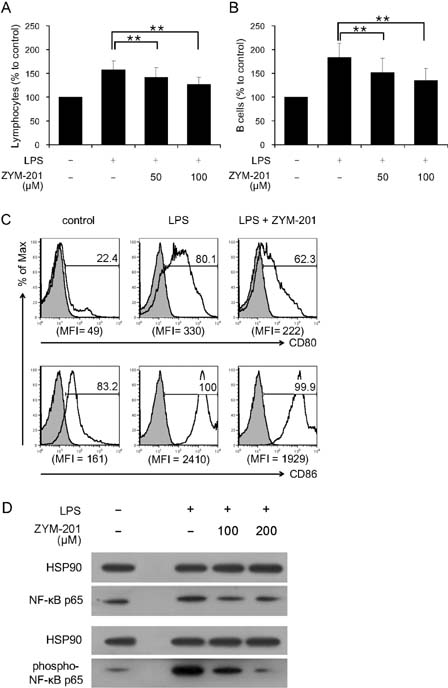
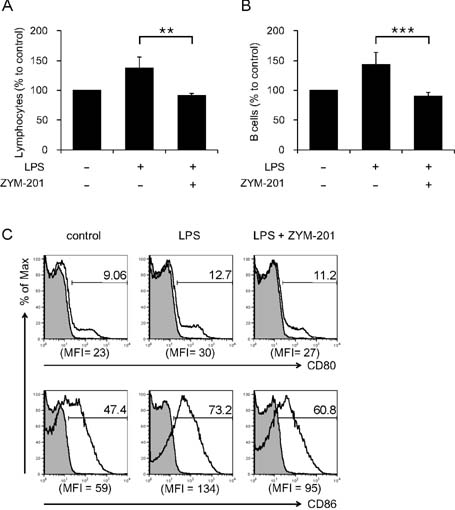
- ZYM-201 is a methyl ester of triterpenoid glycoside from Sanguisorba officinalis which has been used for treatment of inflammatory and metabolic diseases. In this study, immunomodulatory effects of ZYM-201 on B cells were examined in vitro and in vivo. When splenocytes were activated with lipopolysaccharide (LPS), the major population which had shown an increase in cell numbers was B cells. However, when the B cells were treated with ZYM-201 after LPS activation, their cell numbers and the expression of major costimulatory molecules, CD80 and CD86, were decreased. Furthermore, the effect of LPS, which induces activation of NF-kappaB, was abolished by ZYM-201: LPS-stimulated B cells showed decrease of phosphorylation after treatment of ZYM-201. The same results were shown in vivo experiments. These results suggest that ZYM-201 may play a role in the modulation of inflammatory responses through inhibiting NF-kappaB activation and downregulating the expression of costimulatory molecules on B cells.
Keyword
MeSH Terms
Figure
Reference
-
1. East J. The effect of certain plant preparations on the fertility of laboratory mammals. 4. Sanguisorba officinalis L. J Endocrinol. 1955; 12:273–276.
Article2. Park KH, Koh D, Kim K, Park J, Lim Y. Antiallergic activity of a disaccharide isolated from Sanguisorba officinalis. Phytother Res. 2004; 18:658–662.
Article3. Goun EA, Petrichenko VM, Solodnikov SU, Suhinina TV, Kline MA, Cunningham G, Nguyen C, Miles H. Anticancer and antithrombin activity of Russian plants. J Ethnopharmacol. 2002; 81:337–342.
Article4. Kim YH, Chung CB, Kim JG, Ko KI, Park SH, Kim JH, Eom SY, Kim YS, Hwang YI, Kim KH. Anti-wrinkle activity of ziyuglycoside I isolated from a Sanguisorba officinalis root extract and its application as a cosmeceutical ingredient. Biosci Biotechnol Biochem. 2008; 72:303–311.
Article5. Ban JY, Nguyen HT, Lee HJ, Cho SO, Ju HS, Kim JY, Bae K, Song KS, Seong YH. Neuroprotective properties of gallic acid from Sanguisorbae radix on amyloid beta protein (25--35)-induced toxicity in cultured rat cortical neurons. Biol Pharm Bull. 2008; 31:149–153.
Article6. Wang Z, Loo WT, Wang N, Chow LW, Wang D, Han F, Zheng X, Chen JP. Effect of Sanguisorba officinalis L on breast cancer growth and angiogenesis. Expert Opin Ther Targets. 2012; 16:Suppl 1. S79–S89.7. Tsukahara K, Moriwaki S, Fujimura T, Takema Y. Inhibitory effect of an extract of Sanguisorba officinalis L. on ultraviolet-B-induced photodamage of rat skin. Biol Pharm Bull. 2001; 24:998–1003.
Article8. Liu X, Cui Y, Yu Q, Yu B. Triterpenoids from Sanguisorba officinalis. Phytochemistry. 2005; 66:1671–1679.9. Mimaki Y, Fukushima M, Yokosuka A, Sashida Y, Furuya S, Sakagami H. Triterpene glycosides from the roots of Sanguisorba officinalis. Phytochemistry. 2001; 57:773–779.
Article10. Cho JY, Yoo ES, Cha BC, Park HJ, Rhee MH, Han YN. The inhibitory effect of triterpenoid glycosides originating from Sanguisorba officinalis on tissue factor activity and the production of TNF-alpha. Planta Med. 2006; 72:1279–1284.
Article11. Choi J, Kim MY, Cha BC, Yoo ES, Yoon K, Lee J, Rho HS, Kim SY, Cho JY. ZYM-201 sodium succinate ameliorates streptozotocin-induced hyperlipidemic conditions. Planta Med. 2012; 78:12–17.
Article12. Choi J, Yu T, Cha BC, Rhee MH, Yoo ES, Kim MY, Lee J, Cho JY. Modulatory effects of ZYM-201 sodium succinate on dietary-induced hyperlipidemic conditions. Pharmazie. 2011; 66:791–797.13. Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008; 42:145–151.
Article14. Alexander C, Rietschel ET. Bacterial lipopolysaccharides and innate immunity. J Endotoxin Res. 2001; 7:167–202.
Article15. Akira S, Sato S. Toll-like receptors and their signaling mechanisms. Scand J Infect Dis. 2003; 35:555–562.
Article16. Buss H, Dorrie A, Schmitz ML, Hoffmann E, Resch K, Kracht M. Constitutive and interleukin-1-inducible phosphorylation of p65 NF-{kappa}B at serine 536 is mediated by multiple protein kinases including I{kappa}B kinase (IKK)-{alpha}, IKK{beta}, IKK{epsilon}, TRAF family member-associated (TANK)-binding kinase 1 (TBK1), and an unknown kinase and couples p65 to TATA-binding protein-associated factor II31-mediated interleukin-8 transcription. J Biol Chem. 2004; 279:55633–55643.
Article17. Suvas S, Singh V, Sahdev S, Vohra H, Agrewala JN. Distinct role of CD80 and CD86 in the regulation of the activation of B cell and B cell lymphoma. J Biol Chem. 2002; 277:7766–7775.
Article18. Ogata H, Su I, Miyake K, Nagai Y, Akashi S, Mecklenbrauker I, Rajewsky K, Kimoto M, Tarakhovsky A. The toll-like receptor protein RP105 regulates lipopolysaccharide signaling in B cells. J Exp Med. 2000; 192:23–29.
Article19. Collins AV, Brodie DW, Gilbert RJ, Iaboni A, Manso-Sancho R, Walse B, Stuart DI, van der Merwe PA, Davis SJ. The interaction properties of costimulatory molecules revisited. Immunity. 2002; 17:201–210.
Article20. Giannoukakis NC, Bonham A, Qian S, Chen Z, Peng L, Harnaha J, Li W, Thomson AW, Fung JJ, Robbins PD, Lu L. Prolongation of cardiac allograft survival using dendritic cells treated with NF-kB decoy oligodeoxyribonucleotides. Mol Ther. 2000; 1:430–437.
Article21. Zhang G, Ghosh S. Toll-like receptor-mediated NF-kappaB activation: a phylogenetically conserved paradigm in innate immunity. J Clin Invest. 2001; 107:13–19.
Article22. Viatour P, Merville MP, Bours V, Chariot A. Phosphorylation of NF-kappaB and IkappaB proteins: implications in cancer and inflammation. Trends Biochem Sci. 2005; 30:43–52.
Article23. Pierce JW, Read MA, Ding H, Luscinskas FW, Collins T. Collins. Salicylates inhibit I kappa B-alpha phosphorylation, endothelial-leukocyte adhesion molecule expression, and neutrophil transmigration. J Immunol. 1996; 156:3961–3969.24. Yin MJ, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature. 1998; 396:77–80.
Article25. Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001; 107:135–142.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Phosphatidylinositol-3-kinase Inhibitor Enhances Nitric Oxide Synthesis and Apoptosis in LPS-Stimulated Macrophages
- The Effect of IFN- and PGE2 in the Production of TNF- by Human PBMC Stimulated with LPS
- Biphasic immunomodulatory effects of ionized biosilica water on the antigen-presenting capability of mouse dendritic cells
- Immunomodulatory Effects of Callophyllis japonica Ethanol Extract on Dendritic Cells
- Oral squamous carcinoma cells stimulated by Porphyromonas gingivalis-derived lipopolysaccharide induce osteoclastogenesis through a paracrine mechanism