Nutr Res Pract.
2017 Aug;11(4):275-280. 10.4162/nrp.2017.11.4.275.
Astaxanthin induces migration in human skin keratinocytes via Rac1 activation and RhoA inhibition
- Affiliations
-
- 1Department of Pharmacology, Faculty of Science, Prince of Songkla University, 15 Hat Yai, Songkhla 90110, Thailand. wanida.su@psu.ac.th
- 2Department of Anatomy, Faculty of Science, Prince of Songkla University, Hat Yai, Songkhla 90110, Thailand.
- 3Department of Fishery Product, Faculty of Fishery, Kasetsart University, Bangkok 10900, Thailand.
- 4Department of Physiology, Faculty of Science, Prince of Songkla University, Hat Yai, Songkhla 90110, Thailand.
- 5Department of Biomedical Sciences, Faculty of Medicine, Prince of Songkla University, Hat Yai, Songkhla 90110, Thailand.
- KMID: 2385465
- DOI: http://doi.org/10.4162/nrp.2017.11.4.275
Abstract
- BACKGROUND/OBJECTIVES
Re-epithelialization has an important role in skin wound healing. Astaxanthin (ASX), a carotenoid found in crustaceans including shrimp, crab, and salmon, has been widely used for skin protection. Therefore, we investigated the effects of ASX on proliferation and migration of human skin keratinocyte cells and explored the mechanism associated with that migration. MATERIAL/METHOD: HaCaT keratinocyte cells were exposed to 0.25-1 µg/mL of ASX. Proliferation of keratinocytes was analyzed by using MTT assays and flow cytometry. Keratinocyte migration was determined by using a scratch wound-healing assay. A mechanism for regulation of migration was explored via immunocytochemistry and western blot analysis.
RESULTS
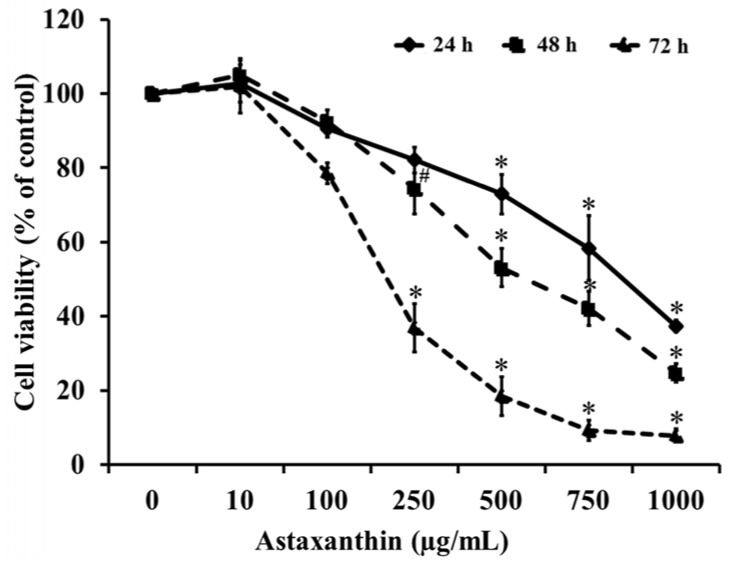
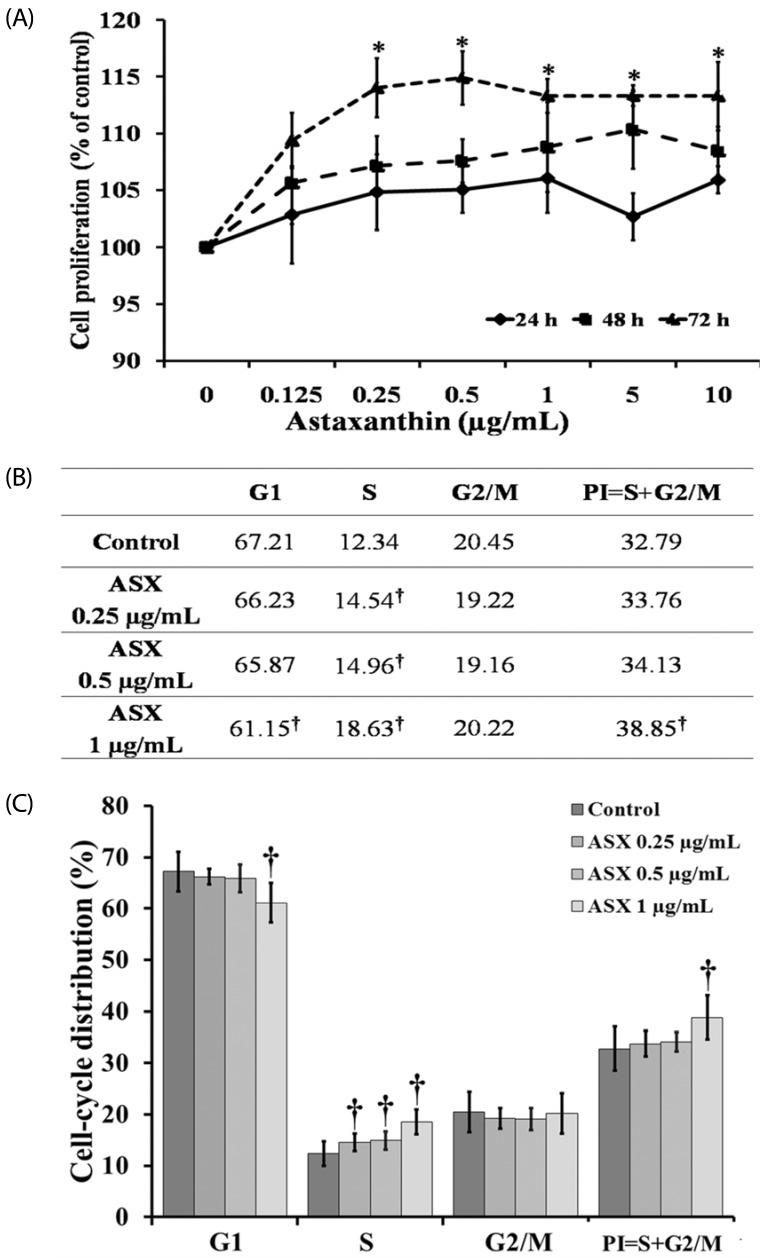
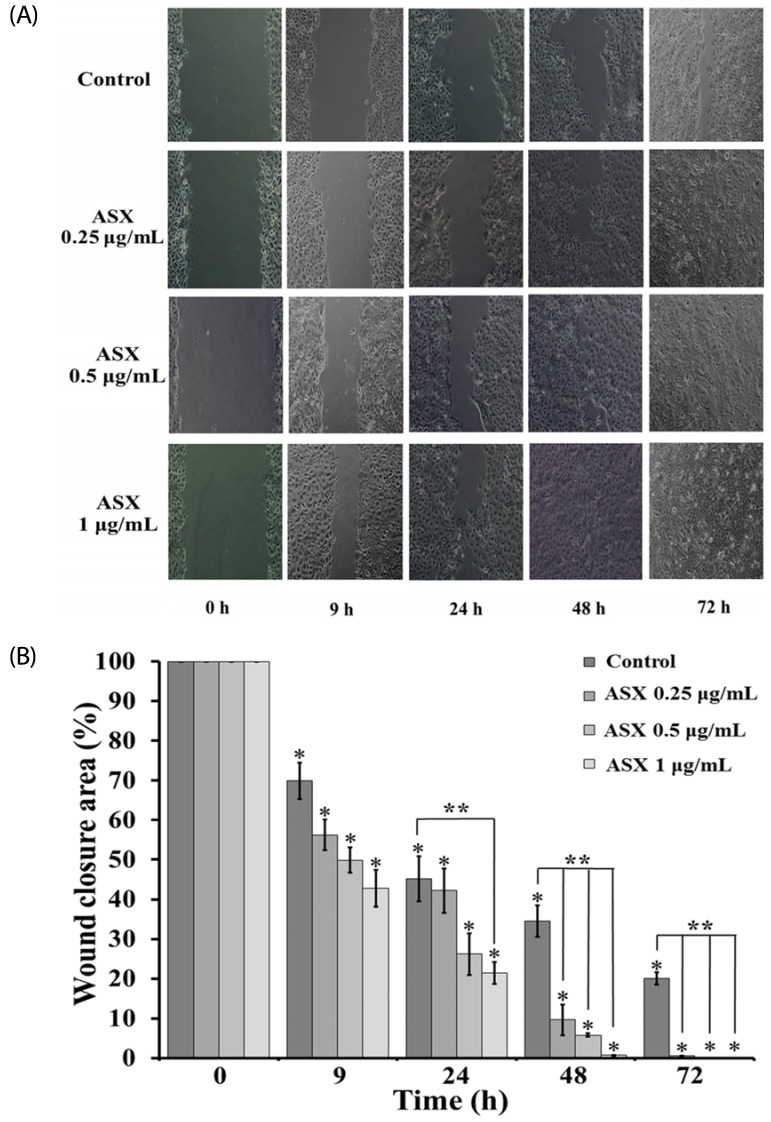
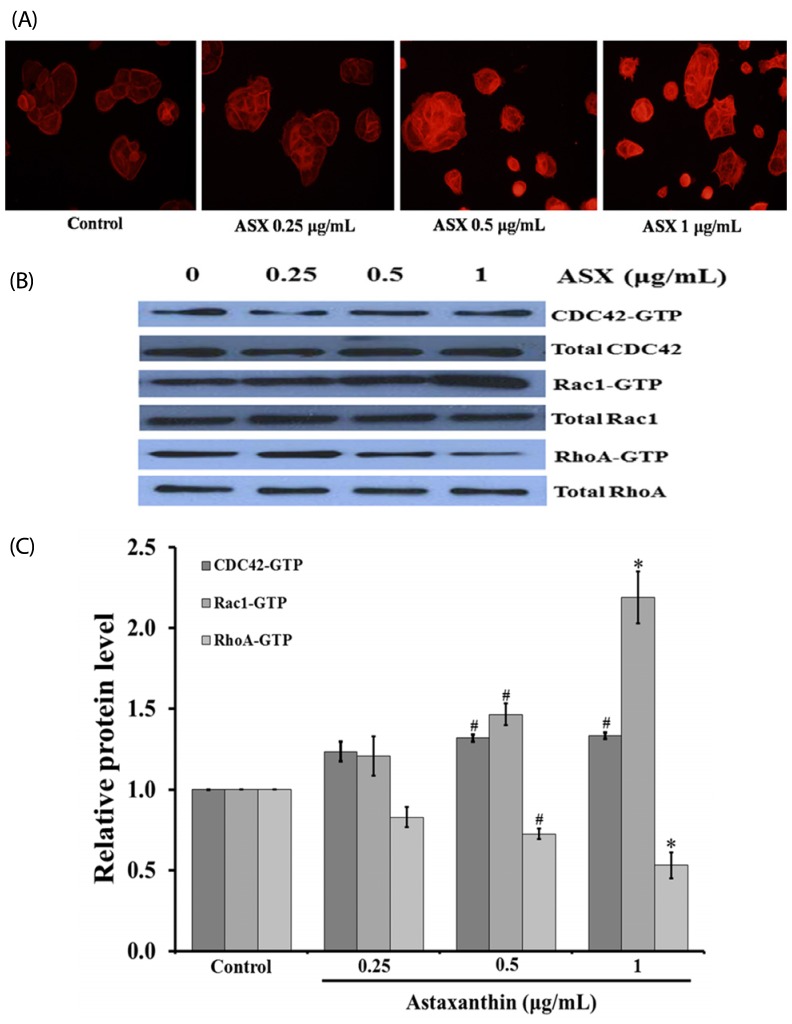
Our results suggest that ASX produces no significant toxicity in human keratinocyte cells. Cell-cycle analysis on ASX-treated keratinocytes demonstrated a significant increase in keratinocyte cell proliferation at the S phase. In addition, ASX increased keratinocyte motility across the wound space in a time-dependent manner. The mechanism by which ASX increased keratinocyte migration was associated with induction of filopodia and formation of lamellipodia, as well as with increased Cdc42 and Rac1 activation and decreased RhoA activation.
CONCLUSIONS
ASX stimulates the migration of keratinocytes through Cdc42, Rac1 activation and RhoA inhibition. ASX has a positive role in the re-epithelialization of wounds. Our results may encourage further in vivo and clinical study into the development of ASX as a potential agent for wound repair.
MeSH Terms
Figure
Reference
-
1. Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008; 453:314–321. PMID: 18480812.
Article2. Wadman M. Scar prevention: the healing touch. Nature. 2005; 436:1079–1080. PMID: 16121148.3. Haase I, Evans R, Pofahl R, Watt FM. Regulation of keratinocyte shape, migration and wound epithelialization by IGF-1- and EGF-dependent signalling pathways. J Cell Sci. 2003; 116:3227–3238. PMID: 12829742.
Article4. Enciso JM, Konecny CM, Karpen HE, Hirschi KK. Endothelial cell migration during murine yolk sac vascular remodeling occurs by means of a Rac1 and FAK activation pathway in vivo. Dev Dyn. 2010; 239:2570–2583. PMID: 20737513.
Article5. Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol. 1996; 12:463–518. PMID: 8970735.
Article6. Sepp KJ, Auld VJ. RhoA and Rac1 GTPases mediate the dynamic rearrangement of actin in peripheral glia. Development. 2003; 130:1825–1835. PMID: 12642488.
Article7. Nobes CD, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J Cell Biol. 1999; 144:1235–1244. PMID: 10087266.
Article8. Hsu CL, Muerdter CP, Knickerbocker AD, Walsh RM, Zepeda-Rivera MA, Depner KH, Sangesland M, Cisneros TB, Kim JY, Sanchez-Vazquez P, Cherezova L, Regan RD, Bahrami NM, Gray EA, Chan AY, Chen T, Rao MY, Hille MB. Cdc42 GTPase and Rac1 GTPase act downstream of p120 catenin and require GTP exchange during gastrulation of zebrafish mesoderm. Dev Dyn. 2012; 241:1545–1561. PMID: 22911626.
Article9. Rottner K, Hall A, Small JV. Interplay between Rac and Rho in the control of substrate contact dynamics. Curr Biol. 1999; 9:640–648. PMID: 10375527.
Article10. Kidd P. Astaxanthin, cell membrane nutrient with diverse clinical benefits and anti-aging potential. Altern Med Rev. 2011; 16:355–364. PMID: 22214255.11. Naguib YM. Antioxidant activities of astaxanthin and related carotenoids. J Agric Food Chem. 2000; 48:1150–1154. PMID: 10775364.
Article12. Ranga Rao A, Baskaran V, Sarada R, Ravishankar GA. In vivo bioavailability and antioxidant activity of carotenoids from microalgal biomass: a repeated dose study. Food Res Int. 2013; 54:711–717.13. Choi SK, Park YS, Choi DK, Chang HI. Effects of astaxanthin on the production of NO and the expression of COX-2 and iNOS in LPS-stimulated BV2 microglial cells. J Microbiol Biotechnol. 2008; 18:1990–1996. PMID: 19131704.14. Kishimoto Y, Tani M, Uto-Kondo H, Iizuka M, Saita E, Sone H, Kurata H, Kondo K. Astaxanthin suppresses scavenger receptor expression and matrix metalloproteinase activity in macrophages. Eur J Nutr. 2010; 49:119–126. PMID: 19784539.
Article15. Kuedo Z, Sangsuriyawong A, Klaypradit W, Tipmanee V, Chonpathompikunlert P. Effects of astaxanthin from Litopenaeus vannamei on carrageenan-induced edema and pain behavior in mice. Molecules. 2016; 21:382. PMID: 27007359.
Article16. Camera E, Mastrofrancesco A, Fabbri C, Daubrawa F, Picardo M, Sies H, Stahl W. Astaxanthin, canthaxanthin and β-carotene differently affect UVA-induced oxidative damage and expression of oxidative stress-responsive enzymes. Exp Dermatol. 2009; 18:222–231. PMID: 18803658.
Article17. Hama S, Takahashi K, Inai Y, Shiota K, Sakamoto R, Yamada A, Tsuchiya H, Kanamura K, Yamashita E, Kogure K. Protective effects of topical application of a poorly soluble antioxidant astaxanthin liposomal formulation on ultraviolet-induced skin damage. J Pharm Sci. 2012; 101:2909–2916. PMID: 22628205.
Article18. Mizuta M, Hirano S, Hiwatashi N, Tateya I, Kanemaru S, Nakamura T, Ito J. Effect of astaxanthin on vocal fold wound healing. Laryngoscope. 2014; 124:E1–E7. PMID: 23686840.
Article19. Praveenkumar R, Gwak R, Kang M, Shim TS, Cho S, Lee J, Oh YK, Lee K, Kim B. Regenerative astaxanthin extraction from a single microalgal (Haematococcus pluvialis) cell using a gold nano-scalpel. ACS Appl Mater Interfaces. 2015; 7:22702–22708. PMID: 26397314.
Article20. Xu Y, Zhang J, Jiang W, Zhang S. Astaxanthin induces angiogenesis through Wnt/β-catenin signaling pathway. Phytomedicine. 2015; 22:744–751. PMID: 26141761.
Article21. Schlessinger K, Hall A, Tolwinski N. Wnt signaling pathways meet Rho GTPases. Genes Dev. 2009; 23:265–277. PMID: 19204114.
Article22. Sangsuriyawong A, Limpawattana M, Siriwan D, Kaewnern P, Klaypradit W. Effects of phospholipid concentration and mixing methods on properties of astaxanthin extract-loaded liposomes. J Agric. 2016; 32:421–433.23. Taksima T, Limpawattana M, Klaypradit W. Astaxanthin encapsulated in beads using ultrasonic atomizer and application in yogurt as evaluated by consumer sensory profile. LWT Food Sci Technol. 2015; 62:431–437.24. Gammone MA, Riccioni G, D'Orazio N. Marine carotenoids against oxidative stress: effects on human health. Mar Drugs. 2015; 13:6226–6246. PMID: 26437420.
Article25. Santoro MM, Gaudino G. Cellular and molecular facets of keratinocyte reepithelization during wound healing. Exp Cell Res. 2005; 304:274–286. PMID: 15707592.
Article26. Harper D, Young A, McNaught C. The physiology of wound healing. Surgery. 2014; 32:445–450.
Article27. Kim JH, Nam SW, Kim BW, Choi W, Lee JH, Kim WJ, Choi YH. Astaxanthin improves stem cell potency via an increase in the proliferation of neural progenitor cells. Int J Mol Sci. 2010; 11:5109–5119. PMID: 21614195.
Article28. Hodge RG, Ridley AJ. Regulating Rho GTPases and their regulators. Nat Rev Mol Cell Biol. 2016; 17:496–510. PMID: 27301673.
Article29. Fukata M, Nakagawa M, Kaibuchi K. Roles of Rho-family GTPases in cell polarisation and directional migration. Curr Opin Cell Biol. 2003; 15:590–597. PMID: 14519394.30. Li J, Zhang S, Soto X, Woolner S, Amaya E. ERK and phosphoinositide 3-kinase temporally coordinate different modes of actin-based motility during embryonic wound healing. J Cell Sci. 2013; 126:5005–5017. PMID: 23986484.
Article31. Zhang Z, Yang M, Chen R, Su W, Li P, Chen S, Chen Z, Chen A, Li S, Hu C. IBP regulates epithelial-to-mesenchymal transition and the motility of breast cancer cells via Rac1, RhoA and Cdc42 signaling pathways. Oncogene. 2014; 33:3374–3382. PMID: 23975422.
Article32. Rørth P. Collective cell migration. Annu Rev Cell Dev Biol. 2009; 25:407–429. PMID: 19575657.
Article33. Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Dev Biol. 2004; 265:23–32. PMID: 14697350.
Article34. Zhao X, Guan JL. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv Drug Deliv Rev. 2011; 63:610–615. PMID: 21118706.
Article35. Salhia B, Rutten F, Nakada M, Beaudry C, Berens M, Kwan A, Rutka JT. Inhibition of Rho-kinase affects astrocytoma morphology, motility, and invasion through activation of Rac1. Cancer Res. 2005; 65:8792–8800. PMID: 16204049.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Astaxanthin Protects Ultraviolet B-Induced Oxidative Stress and Apoptosis in Human Keratinocytes via Intrinsic Apoptotic Pathway
- Rac1 inhibition protects the kidney against kidney ischemia/ reperfusion through the inhibition of macrophage migration
- Inhibitory effect of capsaicin on B16-F10 melanoma cell migration via the phosphatidylinositol 3-kinase/Akt/Rac1 signal pathway
- Helicobacter pylori Eradication Can Reverse Rho GTPase Expression in Gastric Carcinogenesis
- Downstream components of RhoA required for signal pathway of superoxide formation during phagocytosis of serum opsonized zymosans in macrophages