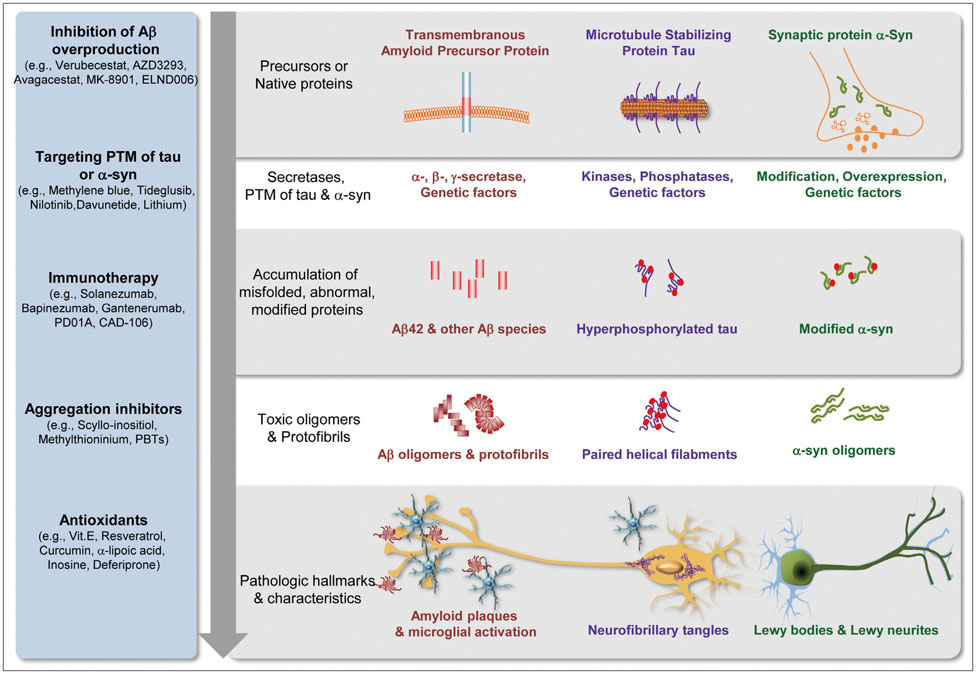
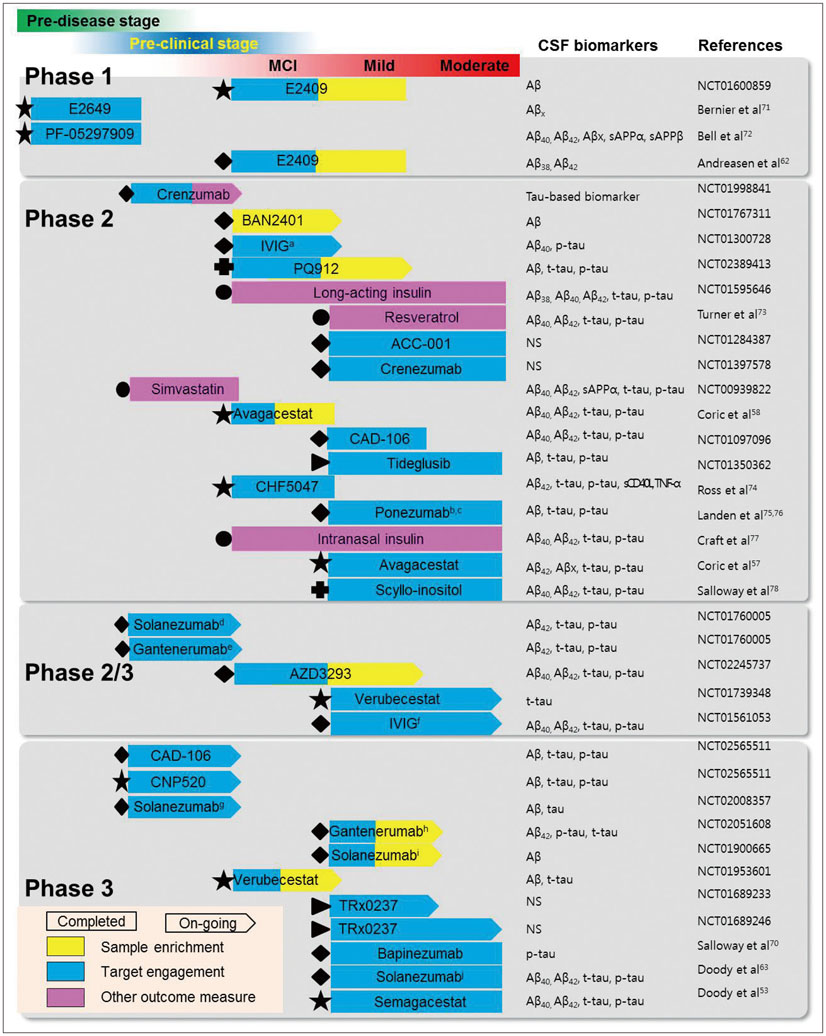
J Clin Neurol.
2016 Oct;12(4):381-392. 10.3988/jcn.2016.12.4.381.
Harnessing Cerebrospinal Fluid Biomarkers in Clinical Trials for Treating Alzheimer's and Parkinson's Diseases: Potential and Challenges
- Affiliations
-
- 1Department of Pharmacology and Medicinal Toxicology Research Center, Incheon, Korea. johykang@inha.ac.kr
- 2Hypoxia-Related Diseases Research Center, Inha University School of Medicine, Incheon, Korea.
- 3Department of Thoracic Surgery, Inha University Hospital, Inha University, Incheon, Korea.
- 4Department of Emergency Medicine, Inje University Ilsan Paik Hospital, Goyang, Korea.
- KMID: 2385100
- DOI: http://doi.org/10.3988/jcn.2016.12.4.381
Abstract
- No disease-modifying therapies (DMT) for neurodegenerative diseases (NDs) have been established, particularly for Alzheimer's disease (AD) and Parkinson's disease (PD). It is unclear why candidate drugs that successfully demonstrate therapeutic effects in animal models fail to show disease-modifying effects in clinical trials. To overcome this hurdle, patients with homogeneous pathologies should be detected as early as possible. The early detection of AD patients using sufficiently tested biomarkers could demonstrate the potential usefulness of combining biomarkers with clinical measures as a diagnostic tool. Cerebrospinal fluid (CSF) biomarkers for NDs are being incorporated in clinical trials designed with the aim of detecting patients earlier, evaluating target engagement, collecting homogeneous patients, facilitating prevention trials, and testing the potential of surrogate markers relative to clinical measures. In this review we summarize the latest information on CSF biomarkers in NDs, particularly AD and PD, and their use in clinical trials. The large number of issues related to CSF biomarker measurements and applications has resulted in relatively few clinical trials on CSF biomarkers being conducted. However, the available CSF biomarker data obtained in clinical trials support the advantages of incorporating CSF biomarkers in clinical trials, even though the data have mostly been obtained in AD trials. We describe the current issues with and ongoing efforts for the use of CSF biomarkers in clinical trials and the plans to harness CSF biomarkers for the development of DMT and clinical routines. This effort requires nationwide, global, and multidisciplinary efforts in academia, industry, and regulatory agencies to facilitate a new era.
Keyword
MeSH Terms
Figure
Reference
-
1. Wimo A, Jönsson L, Bond J, Prince M, Winblad B. Alzheimer Disease International. The worldwide economic impact of dementia 2010. Alzheimers Dement. 2013; 9:1–11.e3.
Article2. Mayeux R, Stern Y. Epidemiology of Alzheimer disease. Cold Spring Harb Perspect Med. 2012; 2:DOI: 10.1101/cshperspect.a006239.
Article3. de Lau LM, Breteler MM. Epidemiology of Parkinson's disease. Lancet Neurol. 2006; 5:525–535.
Article4. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDSADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984; 34:939–944.
Article5. Blacker D, Albert MS, Bassett SS, Go RC, Harrell LE, Folstein MF. Reliability and validity of NINCDS-ADRDA criteria for Alzheimer's disease. The National Institute of Mental Health Genetics Initiative. Arch Neurol. 1994; 51:1198–1204.
Article6. Buchhave P, Minthon L, Zetterberg H, Wallin AK, Blennow K, Hansson O. Cerebrospinal fluid levels of β-amyloid 1-42, but not of tau, are fully changed already 5 to 10 years before the onset of Alzheimer dementia. Arch Gen Psychiatry. 2012; 69:98–106.
Article7. Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, et al. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med. 2012; 367:795–804.
Article8. Davies L, Wolska B, Hilbich C, Multhaup G, Martins R, Simms G, et al. A4 amyloid protein deposition and the diagnosis of Alzheimer's disease: prevalence in aged brains determined by immunocytochemistry compared with conventional neuropathologic techniques. Neurology. 1988; 38:1688–1693.
Article9. Bezard E, Gross CE, Brotchie JM. Presymptomatic compensation in Parkinson's disease is not dopamine-mediated. Trends Neurosci. 2003; 26:215–221.
Article10. de la Fuente-Fernández R. Role of DaTSCAN and clinical diagnosis in Parkinson disease. Neurology. 2012; 78:696–701.
Article11. Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001; 69:89–95.12. Peskind ER, Riekse R, Quinn JF, Kaye J, Clark CM, Farlow MR, et al. Safety and acceptability of the research lumbar puncture. Alzheimer Dis Assoc Disord. 2005; 19:220–225.
Article13. Shaw LM, Korecka M, Clark CM, Lee VM, Trojanowski JQ. Biomarkers of neurodegeneration for diagnosis and monitoring therapeutics. Nat Rev Drug Discov. 2007; 6:295–303.
Article14. Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004; 10 Suppl. S10–S17.
Article15. Selkoe DJ. Cell biology of protein misfolding: the examples of Alzheimer's and Parkinson's diseases. Nat Cell Biol. 2004; 6:1054–1061.
Article16. Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002; 297:353–356.
Article17. Vigo-Pelfrey C, Seubert P, Barbour R, Blomquist C, Lee M, Lee D, et al. Elevation of microtubule-associated protein tau in the cerebrospinal fluid of patients with Alzheimer's disease. Neurology. 1995; 45:788–793.
Article18. Klein WL, Stine WB Jr, Teplow DB. Small assemblies of unmodified amyloid beta-protein are the proximate neurotoxin in Alzheimer's disease. Neurobiol Aging. 2004; 25:569–580.
Article19. Pyykkö OT, Lumela M, Rummukainen J, Nerg O, Seppälä TT, Herukka SK, et al. Cerebrospinal fluid biomarker and brain biopsy findings in idiopathic normal pressure hydrocephalus. PLoS One. 2014; 9:e91974.
Article20. Strozyk D, Blennow K, White LR, Launer LJ. CSF Abeta 42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology. 2003; 60:652–656.
Article21. Hampel H, Bürger K, Teipel SJ, Bokde AL, Zetterberg H, Blennow K. Core candidate neurochemical and imaging biomarkers of Alzheimer's disease. Alzheimers Dement. 2008; 4:38–48.
Article22. Blennow K, Hampel H. CSF markers for incipient Alzheimer's disease. Lancet Neurol. 2003; 2:605–613.
Article23. Tapiola T, Overmyer M, Lehtovirta M, Helisalmi S, Ramberg J, Alafuzoff I, et al. The level of cerebrospinal fluid tau correlates with neurofibrillary tangles in Alzheimer's disease. Neuroreport. 1997; 8:3961–3963.
Article24. Snider BJ, Fagan AM, Roe C, Shah AR, Grant EA, Xiong C, et al. Cerebrospinal fluid biomarkers and rate of cognitive decline in very mild dementia of the Alzheimer type. Arch Neurol. 2009; 66:638–645.
Article25. Kester MI, van der Vlies AE, Blankenstein MA, Pijnenburg YA, van Elk EJ, Scheltens P, et al. CSF biomarkers predict rate of cognitive decline in Alzheimer disease. Neurology. 2009; 73:1353–1358.
Article26. Stomrud E, Hansson O, Blennow K, Minthon L, Londos E. Cerebrospinal fluid biomarkers predict decline in subjective cognitive function over 3 years in healthy elderly. Dement Geriatr Cogn Disord. 2007; 24:118–124.
Article27. Tapiola T, Alafuzoff I, Herukka SK, Parkkinen L, Hartikainen P, Soininen H. Cerebrospinal fluid {beta}-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol. 2009; 66:382–389.28. Kang JH, Korecka M, Toledo JB, Trojanowski JQ, Shaw LM. Clinical utility and analytical challenges in measurement of cerebrospinal fluid amyloid-β(1-42) and τ proteins as Alzheimer disease biomarkers. Clin Chem. 2013; 59:903–916.
Article29. Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, et al. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol. 2009; 65:403–413.
Article30. Vanderstichele H, Bibl M, Engelborghs S, Le Bastard N, Lewczuk P, Molinuevo JL, et al. Standardization of preanalytical aspects of cerebrospinal fluid biomarker testing for Alzheimer's disease diagnosis: a consensus paper from the Alzheimer's Biomarkers Standardization Initiative. Alzheimers Dement. 2012; 8:65–73.
Article31. Fagan AM, Perrin RJ. Upcoming candidate cerebrospinal fluid biomarkers of Alzheimer's disease. Biomark Med. 2012; 6:455–476.
Article32. Marquié M, Normandin MD, Vanderburg CR, Costantino IM, Bien EA, Rycyna LG, et al. Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann Neurol. 2015; 78:787–800.
Article33. Ishiki A, Okamura N, Furukawa K, Furumoto S, Harada R, Tomita N, et al. Longitudinal assessment of tau pathology in patients with Alzheimer's disease using [18F]THK-5117 positron emission tomography. PLoS One. 2015; 10:e0140311.
Article34. Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997; 388:839–840.35. Lashuel HA, Petre BM, Wall J, Simon M, Nowak RJ, Walz T, et al. Alpha-synuclein, especially the Parkinson's disease-associated mutants, forms pore-like annular and tubular protofibrils. J Mol Biol. 2002; 322:1089–1102.
Article36. Mollenhauer B, Locascio JJ, Schulz-Schaeffer W, Sixel-Döring F, Trenkwalder C, Schlossmacher MG. α-Synuclein and tau concentrations in cerebrospinal fluid of patients presenting with parkinsonism: a cohort study. Lancet Neurol. 2011; 10:230–240.
Article37. Mollenhauer B, Trautmann E, Taylor P, Manninger P, Sixel-Döring F, Ebentheuer J, et al. Total CSF α-synuclein is lower in de novo Parkinson patients than in healthy subjects. Neurosci Lett. 2013; 532:44–48.
Article38. Parnetti L, Chiasserini D, Persichetti E, Eusebi P, Varghese S, Qureshi MM, et al. Cerebrospinal fluid lysosomal enzymes and alpha-synuclein in Parkinson's disease. Mov Disord. 2014; 29:1019–1027.
Article39. Kang JH, Irwin DJ, Chen-Plotkin AS, Siderowf A, Caspell C, Coffey CS, et al. Association of cerebrospinal fluid β-amyloid 1-42, T-tau, Ptau181, and α-synuclein levels with clinical features of drug-naive patients with early Parkinson disease. JAMA Neurol. 2013; 70:1277–1287.
Article40. Shi M, Bradner J, Hancock AM, Chung KA, Quinn JF, Peskind ER, et al. Cerebrospinal fluid biomarkers for Parkinson disease diagnosis and progression. Ann Neurol. 2011; 69:570–580.
Article41. Parkinson Progression. The Parkinson Progression Marker Initiative (PPMI). Prog Neurobiol. 2011; 95:629–635.42. Hong Z, Shi M, Chung KA, Quinn JF, Peskind ER, Galasko D, et al. DJ-1 and alpha-synuclein in human cerebrospinal fluid as biomarkers of Parkinson's disease. Brain. 2010; 133(Pt 3):713–726.
Article43. Kim D, Paik JH, Shin DW, Kim HS, Park CS, Kang JH. What is the clinical significance of cerebrospinal fluid biomarkers in Parkinson's disease? Is the significance diagnostic or prognostic? Exp Neurobiol. 2014; 23:352–364.
Article44. Levy G, Tang MX, Louis ED, Côté LJ, Alfaro B, Mejia H, et al. The association of incident dementia with mortality in PD. Neurology. 2002; 59:1708–1713.
Article45. Buter TC, van den Hout A, Matthews FE, Larsen JP, Brayne C, Aarsland D. Dementia and survival in Parkinson disease: a 12-year population study. Neurology. 2008; 70:1017–1022.
Article46. Siderowf A, Xie SX, Hurtig H, Weintraub D, Duda J, Chen-Plotkin A, et al. CSF amyloid {beta} 1-42 predicts cognitive decline in Parkinson disease. Neurology. 2010; 75:1055–1061.
Article47. Alves G, Lange J, Blennow K, Zetterberg H, Andreasson U, Førland MG, et al. CSF Aβ42 predicts early-onset dementia in Parkinson disease. Neurology. 2014; 82:1784–1790.
Article48. Mattsson N, Rajendran L, Zetterberg H, Gustavsson M, Andreasson U, Olsson M, et al. BACE1 inhibition induces a specific cerebrospinal fluid β-amyloid pattern that identifies drug effects in the central nervous system. PLoS One. 2012; 7:e31084.
Article49. Forman M, Palcza J, Tseng J, Leempoels J, Ramael S, Han D, et al. The novel BACE inhibitor MK-8931 dramatically lowers cerebrospinal fluid aβ peptides in healthy subjects following single- and multiple-dose administration. Alzheimers Dement. 2012; 8:4 suppl. P704. DOI: 10.1016/j.jalz.2012.05.1900.50. Forman M, Kleijn H, Dockendorf M, Palcza J, Tseng J, Canales C, et al. The novel BACE inhibitor MK-8931 dramatically lowers CSF beta-amyloid in patients with mild-to-moderate Alzheimer's disease. Alzheimers Dement. 2013; 9:4 suppl. P139. DOI: 10.1016/j.jalz.2013.04.083.51. Höglund K, Salter H, Zetterberg H, Andreason U, Olsson T, Alexander R, et al. Monitoring the soluble amyloid precursor protein alpha (SAPPA) and beta (SAPPB) fragments in plasma and CSF from healthy individuals treated with bace inhibitor AZD3293 in a multiple ascending dose study: pharmacokinetic and pharmacodynamic correlate. Alzheimers Dement. 2014; 10:4 suppl. P447. DOI: 10.1016/j.jalz.2014.05.605.52. Alexander R, Budd S, Russell M, Kugler A, Cebers G, Ye N, et al. AZD3293 A novel BACE1 inhibitor: Safety, tolerability, and effects on plasma and CSF aβ peptides following single- and multiple-dose administration. Neurobiol Aging. 2014; 35:suppl 1. S2.
Article53. Doody RS, Raman R, Farlow M, Iwatsubo T, Vellas B, Joffe S, et al. A phase 3 trial of semagacestat for treatment of Alzheimer's disease. N Engl J Med. 2013; 369:341–350.
Article54. Fleisher AS, Raman R, Siemers ER, Becerra L, Clark CM, Dean RA, et al. Phase 2 safety trial targeting amyloid beta production with a gamma-secretase inhibitor in Alzheimer disease. Arch Neurol. 2008; 65:1031–1038.55. Siemers ER, Quinn JF, Kaye J, Farlow MR, Porsteinsson A, Tariot P, et al. Effects of a gamma-secretase inhibitor in a randomized study of patients with Alzheimer disease. Neurology. 2006; 66:602–604.
Article56. Siemers E, Skinner M, Dean RA, Gonzales C, Satterwhite J, Farlow M, et al. Safety, tolerability, and changes in amyloid beta concentrations after administration of a gamma-secretase inhibitor in volunteers. Clin Neuropharmacol. 2005; 28:126–132.
Article57. Coric V, van Dyck CH, Salloway S, Andreasen N, Brody M, Richter RW, et al. Safety and tolerability of the γ-secretase inhibitor avagacestat in a phase 2 study of mild to moderate Alzheimer disease. Arch Neurol. 2012; 69:1430–1440.
Article58. Coric V, Salloway S, van Dyck CH, Dubois B, Andreasen N, Brody M, et al. Targeting prodromal Alzheimer disease with avagacestat: a randomized clinical trial. JAMA Neurol. 2015; 72:1324–1333.
Article59. Dockens R, Wang JS, Castaneda L, Sverdlov O, Huang SP, Slemmon R, et al. A placebo-controlled, multiple ascending dose study to evaluate the safety, pharmacokinetics and pharmacodynamics of avagacestat (BMS-708163) in healthy young and elderly subjects. Clin Pharmacokinet. 2012; 51:681–693.
Article60. Holland D, McEvoy LK, Desikan RS, Dale AM. Enrichment and stratification for predementia Alzheimer disease clinical trials. PLoS One. 2012; 7:e47739.
Article61. Blennow K, Zetterberg H. Use of CSF biomarkers in Alzheimer's disease clinical trials. J Nutr Health Aging. 2009; 13:358–361.
Article62. Andreasen N, Simeoni M, Ostlund H, Lisjo PI, Fladby T, Loercher AE, et al. First administration of the Fc-attenuated anti-β amyloid antibody GSK933776 to patients with mild Alzheimer's disease: a randomized, placebo-controlled study. PLoS One. 2015; 10:e0098153.
Article63. Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer's disease. N Engl J Med. 2014; 370:311–321.
Article64. Siemers ER, Sundell KL, Carlson C, Case M, Sethuraman G, Liu-Seifert H, et al. Phase 3 solanezumab trials: secondary outcomes in mild Alzheimer's disease patients. Alzheimers Dement. 2016; 12:110–120.
Article65. Vellas B, Carrillo MC, Sampaio C, Brashear HR, Siemers E, Hampel H, et al. Designing drug trials for Alzheimer's disease: what we have learned from the release of the phase III antibody trials: a report from the EU/US/CTAD Task Force. Alzheimers Dement. 2013; 9:438–444.
Article66. Reiman EM, Langbaum JB, Fleisher AS, Caselli RJ, Chen K, Ayutyanont N, et al. Alzheimer's Prevention Initiative: a plan to accelerate the evaluation of presymptomatic treatments. J Alzheimers Dis. 2011; 26:Suppl 3. 321–329.
Article67. Ayutyanont N, Langbaum JB, Hendrix SB, Chen K, Fleisher AS, Friesenhahn M, et al. The Alzheimer's prevention initiative composite cognitive test score: sample size estimates for the evaluation of preclinical Alzheimer's disease treatments in presenilin 1 E280A mutation carriers. J Clin Psychiatry. 2014; 75:652–660.68. Mills SM, Mallmann J, Santacruz AM, Fuqua A, Carril M, Aisen PS, et al. Preclinical trials in autosomal dominant AD: implementation of the DIAN-TU trial. Rev Neurol (Paris). 2013; 169:737–743.
Article69. Kang JH, Ryoo NY, Shin DW, Trojanowski JQ, Shaw LM. Role of cerebrospinal fluid biomarkers in clinical trials for Alzheimer's disease modifying therapies. Korean J Physiol Pharmacol. 2014; 18:447–456.
Article70. Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer's disease. N Engl J Med. 2014; 370:322–333.
Article71. Bernier F, Sato Y, Matijevic M, Desmond H, McGrath S, Burns L, et al. Clinical study of E2609, a novel BACE1 inhibitor, demonstrates target engagement and inhibition of BACE1 activity in CSF. Alzheimers Dement. 2013; 9:4 suppl. P886. DOI: 10.1016/j.jalz.2013.08.244.72. Bell J, O'Neill B, Brodney M, Hajos-Korcsok E, Lu Y, Riddell D, et al. A novel BACE inhibitor (PF-05297909): a two-part adaptive design to evaluate safety, pharmacokinetics and pharmacodynamics for modifying beta-amyloid in a first-in-human study. Alzheimers Dement. 2013; 9:4 suppl. P287. DOI: 10.1016/j.jalz.2013.05.578.73. Turner RS, Thomas RG, Craft S, van Dyck CH, Mintzer J, Reynolds BA, et al. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology. 2015; 85:1383–1391.
Article74. Ross J, Sharma S, Winston J, Nunez M, Bottini G, Franceschi M, et al. CHF5074 reduces biomarkers of neuroinflammation in patients with mild cognitive impairment: a 12-week, double-blind, placebocontrolled study. Curr Alzheimer Res. 2013; 10:742–753.
Article75. Landen J, Cohen S, Billing C, Cronenberger C, Styren S, Burstein A, et al. Safety, efficacy, pharmacokinetics and pharmacodynamics of multiple doses of ponezumab in subjects with mild-to-moderate Alzheimer's disease. Alzheimers Dement. 2012; 8:4 suppl. P708. DOI: 10.1016/j.jalz.2012.05.1913.76. Landen J, Andreasen N, Cronenberger C, Schwartz P, Börjesson-Hanson A, Östlund H, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics of monthly and quarterly doses of ponezumab (PF-04360365) in subjects with mild-to-moderate Alzheimer's disease. Alzheimers Dement. 2012; 8:4 suppl. P708. DOI: 10.1016/j.jalz.2012.05.1914.77. Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2012; 69:29–38.
Article78. Salloway S, Sperling R, Keren R, Porsteinsson AP, van Dyck CH, Tariot PN, et al. A phase 2 randomized trial of ELND005, scyllo-inositol, in mild to moderate Alzheimer disease. Neurology. 2011; 77:1253–1262.
Article79. Parkinson Study, Schwarzschild MA, Ascherio A, Beal MF, Cudkowicz ME, Curhan GC, et al. Inosine to increase serum and cerebrospinal fluid urate in Parkinson disease: a randomized clinical trial. JAMA Neurol. 2014; 71:141–150.80. Devos D, Moreau C, Devedjian JC, Kluza J, Petrault M, Laloux C, et al. Targeting chelatable iron as a therapeutic modality in Parkinson's disease. Antioxid Redox Signal. 2014; 21:195–210.
Article81. Paul G, Zachrisson O, Varrone A, Almqvist P, Jerling M, Lind G, et al. Safety and tolerability of intracerebroventricular PDGF-BB in Parkinson's disease patients. J Clin Invest. 2015; 125:1339–1346.
Article82. Boxer AL, Lang AE, Grossman M, Knopman DS, Miller BL, Schneider LS, et al. Davunetide in patients with progressive supranuclear palsy: a randomised, double-blind, placebo-controlled phase 2/3 trial. Lancet Neurol. 2014; 13:676–685.
Article83. Coart E, Barrado LG, Vanderstichele H, Burzykowski T. The confidence level of established cut-off values for CSF Alzheimer's diseasespecific biomarkers. Alzheimers Dement. 2015; 11:7 suppl. P298. DOI: 10.1016/j.jalz.2015.07.410.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cerebrospinal Fluid Amyloid β1-42, Tau, and Alpha-Synuclein Predict the Heterogeneous Progression of Cognitive Dysfunction in Parkinson's Disease
- Urinary Biomarkers for Neurodegenerative Diseases
- Logopenic Progressive Aphasia Revealing Positive Cerebrospinal Fluid Biomarkers for Alzheimer's Disease
- What is the Clinical Significance of Cerebrospinal Fluid Biomarkers in Parkinson's disease? Is the Significance Diagnostic or Prognostic?
- Role of Cerebrospinal Fluid Biomarkers in Clinical Trials for Alzheimer's Disease Modifying Therapies