J Korean Neurosurg Soc.
2017 May;60(3):348-354. 10.3340/jkns.2016.0707.002.
Difference in Spinal Fusion Process in Osteopenic and Nonosteopenic Living Rat Models Using Serial Microcomputed Tomography
- Affiliations
-
- 1Department of Neurosurgery, Seoul National University Boramae Medical Center, Seoul, Korea.
- 2Department of Neurosurgery, Seoul National University College of Medicine, Seoul, Korea. chungc@snu.ac.kr
- 3Clinical Research Institute, Seoul National University Hospital, Seoul, Korea.
- 4Department of Brain and Cognitive Sciences, Seoul National University College of Natural Sciences, Seoul, Korea.
- KMID: 2382770
- DOI: http://doi.org/10.3340/jkns.2016.0707.002
Abstract
OBJECTIVE
To identify and investigate differences in spinal fusion between the normal and osteopenic spine in a rat model.
METHODS
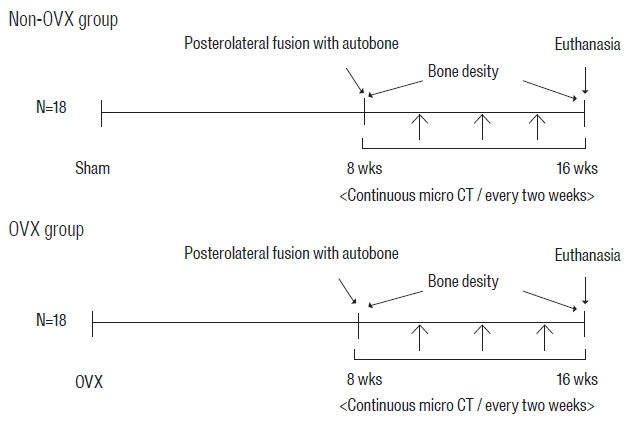
Female Sprague Dawley rats underwent either an ovariectomy (OVX) or sham operation and were randomized into two groups: non-OVX group and OVX group. Eight weeks after OVX, unilateral lumbar spinal fusion was performed using autologous iliac bone. Bone density (BD) was measured 2 days and 8 weeks after fusion surgery. Microcomputed tomography was used to evaluate the process of bone fusion every two weeks for 8 weeks after fusion surgery. The fusion rate, fusion process, and bone volume parameters of fusion bed were compared between the two groups.
RESULTS
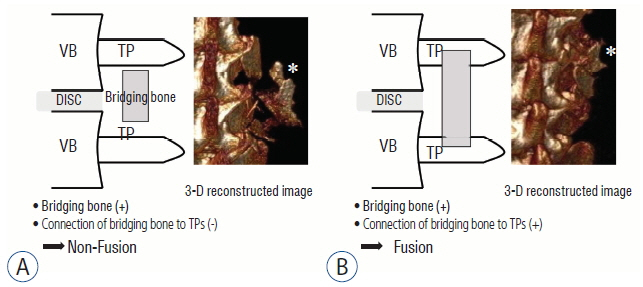
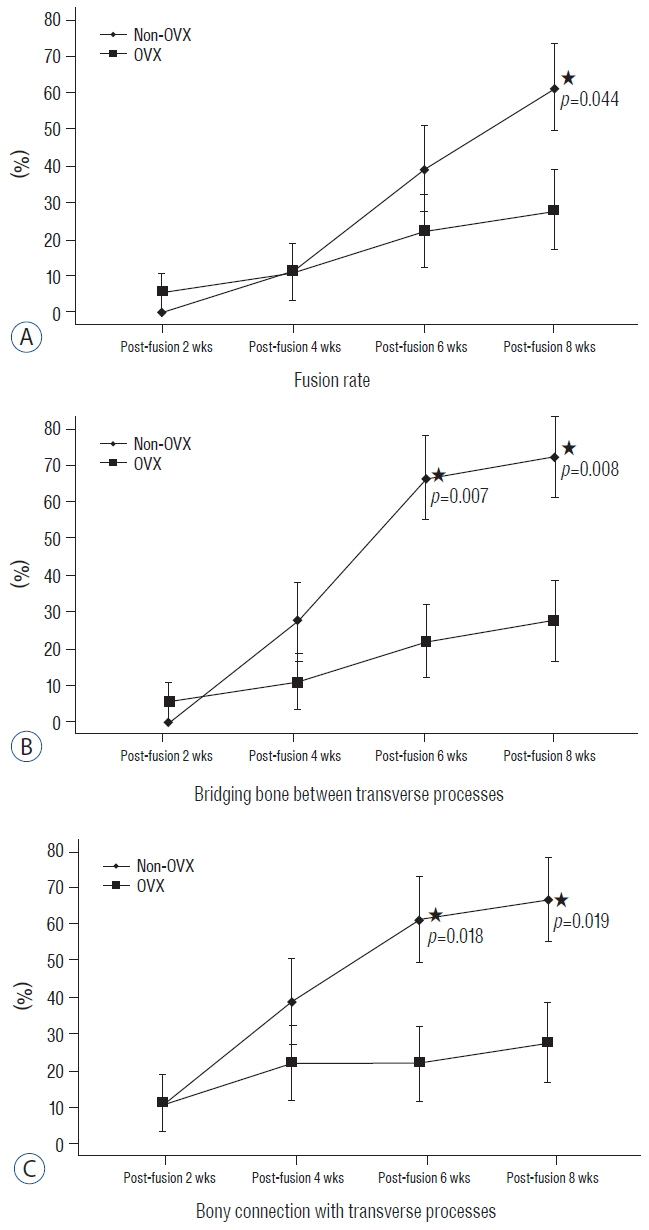
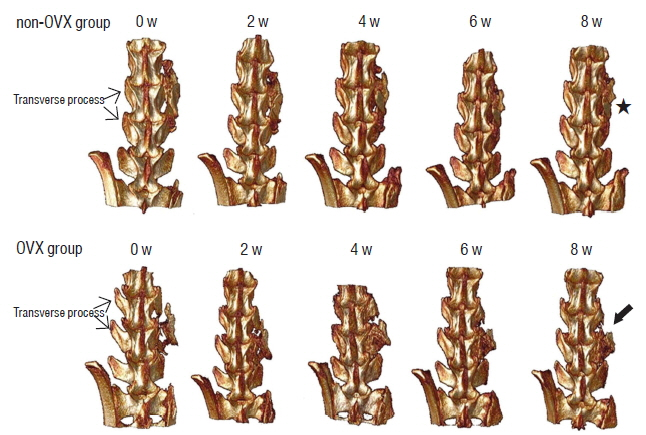
BD was significantly higher in the non-OVX group than in the OVX group 2 days and 8 weeks after fusion surgery. The fusion rate in the non-OVX group was higher than that in the OVX group 8 weeks after surgery (p=0.044). The bony connection of bone fragments with transverse processes and bone formation between transverse processes in non-OVX group were significantly superior to those of OVX group from 6 weeks after fusion surgery. The compactness and bone maturation of fusion bed in non-OVX were prominent compared with the non-OVX group.
CONCLUSION
The fusion rate in OVX group was inferior to non-OVX group at late stage after fusion surgery. Bone maturation of fusion bed in the OVX group was inferior compared with the non-OVX group. Fusion enhancement strategies at early stage may be needed to patients with osteoporosis who need spine fusion surgery.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Aldini NN, Fini M, Giavaresi G, Giardino R, Greggi T, Parisini P. Pedicular fixation in the osteoporotic spine: a pilot in vivo study on long-term ovariectomized sheep. J Orthop Res. 20:1217–1224. 2002.
Article2. Bae HW, Zhao L, Kanim LE, Wong P, Marshall D, Delamarter RB. Bone marrow enhances the performance of rhBMP-2 in spinal fusion: a rodent model. J Bone Joint Surg Am. 95:338–347. 2013.3. Boden SD. Biology of lumbar spine fusion and use of bone graft substitutes: present, future, and next generation. Tissue Eng. 6:383–399. 2000.
Article4. Boden SD, Schimandle JH, Hutton WC. An experimental lumbar intertransverse process spinal fusion model. Radiographic, histologic, and biomechanical healing characteristics. Spine (Phila Pa 1976). 20:412–420. 1995.5. Boden SD, Schimandle JH, Hutton WC, Chen MI. 1995 Volvo Award in basic sciences. The use of an osteoinductive growth factor for lumbar spinal fusion Part I: Biology of spinal fusion. Spine (Phila Pa 1976). 20:2626–2632. 1995.6. Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 25:1468–1486. 2010.
Article7. Bridwell KH, Sedgewick TA, O’Brien MF, Lenke LG, Baldus C. The role of fusion and instrumentation in the treatment of degenerative spondylolisthesis with spinal stenosis. J Spinal Disord. 6:461–472. 1993.
Article8. Coe JD, Warden KE, Herzig MA, McAfee PC. Influence of bone mineral density on the fixation of thoracolumbar implants. A comparative study of transpedicular screws, laminar hooks, and spinous process wires. Spine (Phila Pa 1976). 15:902–907. 1990.
Article9. Kamoda H, Ohtori S, Ishikawa T, Miyagi M, Arai G, Suzuki M, et al. The effect of platelet-rich plasma on posterolateral lumbar fusion in a rat model. J Bone Joint Surg Am. 95:1109–1116. 2013.
Article10. Moazzaz P, Gupta MC, Gilotra MM, Gilotra MN, Maitra S, Theerajunyaporn T, et al. Estrogen-dependent actions of bone morphogenetic protein-7 on spine fusion in rats. Spine (Phila Pa 1976). 30:1706–1711. 2005.
Article11. Nakao S, Minamide A, Kawakami M, Boden SD, Yoshida M. The influence of alendronate on spine fusion in an osteoporotic animal model. Spine (Phila Pa 1976). 36:1446–1452. 2011.
Article12. Namkung-Matthai H, Appleyard R, Jansen J, Hao Lin J, Maastricht S, Swain M, et al. Osteoporosis influences the early period of fracture healing in a rat osteoporotic model. Bone. 28:80–86. 2001.
Article13. Omi N, Ezawa I. Animal models for bone and joint disease. Low calcium diet-induced rat model of osteoporosis. Clin Calcium. 21:173–180. 2011.14. Park SB, Chung CK. Strategies of spinal fusion on osteoporotic spine. J Korean Neurosurg Soc. 49:317–322. 2011.
Article15. Park SB, Kim CH, Hong M, Yang HJ, Chung CK. Effect of a selective estrogen receptor modulator on bone formation in osteoporotic spine fusion using an ovariectomized rat model. Spine J. 16:72–81. 2016.
Article16. Schindeler A, McDonald MM, Bokko P, Little DG. Bone remodeling during fracture repair: the cellular picture. Semin Cell Dev Biol. 19:459–466. 2008.
Article17. Takahata M, Ito M, Abe Y, Abumi K, Minami A. The effect of anti-resorptive therapies on bone graft healing in an ovariectomized rat spinal arthrodesis model. Bone. 43:1057–1066. 2008.
Article18. Toribatake Y, Hutton WC, Tomita K, Boden SD. Vascularization of the fusion mass in a posterolateral intertransverse process fusion. Spine (Phila Pa 1976). 23:1149–1154. 1998.
Article19. Wiltse LL, Spencer CW. New uses and refinements of the paraspinal approach to the lumbar spine. Spine (Phila Pa 1976). 13:696–706. 1988.
Article20. Xu SW, Yu R, Zhao GF, Wang JW. Early period of fracture healing in ovariectomized rats. Chin J Traumatol. 6:160–166. 2003.21. Zipfel GJ, Guiot BH, Fessler RG. Bone grafting. Neurosurg Focus. 14:e8. 2003.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Hyaluronate-Carboxymethyl Cellulose on Bone Graft Substitute Healing in a Rat Spinal Fusion Model
- Animal Models of Orthopedic Research: A Spinal Fusion Model
- Influence of Alendronate and Endplate Degeneration to Single Level Posterior Lumbar Spinal Interbody Fusion
- The Effect of Risedronate on Posterior Lateral Spinal Fusion in a Rat Model
- Clinical and Radiological Outcomes of Segmental Spinal Fusion in Transforaminal Lumbar Interbody Fusion with Spinous Process Tricortical Autograft