Anticoagulation Therapy during Extracorporeal Membrane Oxygenator Support in Pediatric Patients
- Affiliations
-
- 1Department of Pediatrics, Chonnam National University Hospital, Chonnam National University Medical School, Gwangju, Korea.
- 2Department of Thoracic and Cardiovascular Surgery, Chonnam National University Hospital, Chonnam National University Medical School, Gwangju, Korea. isjeong1201@gmail.com
- 3Extracorporeal Life Support Organization, Asia-Pacific Chapter, Ann Arbor, MI, USA.
- KMID: 2379283
- DOI: http://doi.org/10.4068/cmj.2017.53.2.110
Abstract
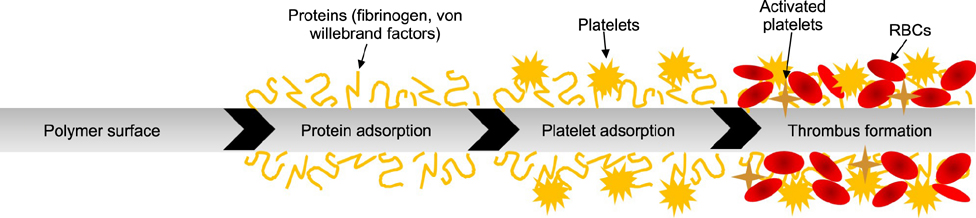
- Extracorporeal membrane oxygenation (ECMO) is a salvage therapy for critically ill patients. Although ECMO is becoming more common, hemorrhagic and thromboembolic complications remain the major causes of death in patients undergoing ECMO treatments. These complications commence upon blood contact with artificial surfaces of the circuit, blood pump, and oxygenator system. Therefore, anticoagulation therapy is required in most cases to prevent these problems. Anticoagulation is more complicated in pediatric patients than in adults, and the foreign surface of ECMO only increases the complexity of systemic anticoagulation. In this review, we discuss the pathophysiology of coagulation, anticoagulants, and monitoring tools in pediatric patients receiving ECMO.
Keyword
MeSH Terms
Figure
Cited by 3 articles
-
Role of Red Cell Distribution Width in the Relationship between Clinical Outcomes and Anticoagulation Response in Patients with Atrial Fibrillation
Ki Hong Lee, Jeong Gwan Cho, Hyung Wook Park, Nam Sik Yoon, Hyung Ki Jeong, Nuri Lee
Chonnam Med J. 2018;54(2):113-120. doi: 10.4068/cmj.2018.54.2.113.Predictive Value of Procalcitonin for Infection and Survival in Adult Cardiogenic Shock Patients Treated with Extracorporeal Membrane Oxygenation
Do Wan Kim, Hwa Jin Cho, Gwan Sic Kim, Sang Yun Song, Kook Joo Na, Sang Gi Oh, Bong Suk Oh, In Seok Jeong
Chonnam Med J. 2018;54(1):48-54. doi: 10.4068/cmj.2018.54.1.48.The role of nafamostat mesilate as a regional anticoagulant during extracorporeal membrane oxygenation
Jae Ha Lee, Jin Han Park, Ji Hoon Jang, Se Hun Kim, Sung Yong Hong, Woon Heo, Dong-Hwan Lee, Hye Sook Choi, Ki Hoon Kim, Hang-Jea Jang
Acute Crit Care. 2022;37(2):177-184. doi: 10.4266/acc.2021.01312.
Reference
-
1. Bartlett RH, Gattinoni L. Current status of extracorporeal life support (ECMO) for cardiopulmonary failure. Minerva Anestesiol. 2010; 76:534–540.2. Thiagarajan RR, Barbaro RP, Rycus PT, McMullan DM, Conrad SA, Fortenberry JD, et al. Extracorporeal life support organization registry international report 2016. ASAIO J. 2017; 63:60–67.
Article3. Werho DK, Pasquali SK, Yu S, Donohue J, Annich GM, Thiagarajan RR, et al. Hemorrhagic complications in pediatric cardiac patients on extracorporeal membrane oxygenation: an analysis of the extracorporeal life support organization registry. Pediatr Crit Care Med. 2015; 16:276–288.
Article4. Wo Y, Brisbois EJ, Bartlett RH, Meyerhoff ME. Recent advances in thromboresistant and antimicrobial polymers for biomedical applications: just say yes to nitric oxide (NO). Biomater Sci. 2016; 4:1161–1183.
Article5. Chenoweth DE, Cooper SW, Hugli TE, Stewart RW, Blackstone EH, Kirklin JW. Complement activation during cardiopulmonary bypass: evidence for generation of C3a and C5a anaphylatoxins. N Engl J Med. 1981; 304:497–503.
Article6. Bruins P, te Velthuis H, Yazdanbakhsh AP, Jansen PG, van Hardevelt FW, de Beaumont EM, et al. Activation of the complement system during and after cardiopulmonary bypass surgery: postsurgery activation involves C-reactive protein and is associated with postoperative arrhythmia. Circulation. 1997; 96:3542–3548.
Article7. Fischer WH, Jagels MA, Hugli TE. Regulation of IL-6 synthesis in human peripheral blood mononuclear cells by C3a and C3a(desArg). J Immunol. 1999; 162:453–459.8. Donnelly RP, Freeman SL, Hayes MP. Inhibition of IL-10 expression by IFN-gamma up-regulates transcription of TNF-alpha in human monocytes. J Immunol. 1995; 155:1420–1427.9. Kawahito K, Nose Y. Hemolysis in different centrifugal pumps. Artif Organs. 1997; 21:323–326.
Article10. Leverett LB, Hellums JD, Alfrey CP, Lynch EC. Red blood cell damage by shear stress. Biophys J. 1972; 12:257–273.
Article11. Hoffman M. A cell-based model of coagulation and the role of factor VIIa. Blood Rev. 2003; 17:Suppl 1. S1–S5.
Article12. Becker RC. Cell-based models of coagulation: a paradigm in evolution. J Thromb Thrombolysis. 2005; 20:65–68.
Article13. Lequier LL, Massicotte P. Anticoagulation and bleeding during ECLS. In : Annich G, editor. ECMO: Extracorporeal Cardiopulmonary Support in Critical Care. 4th ed. Ann Arbor: Extracorporeal Life Support Organization;2012. p. 157–170.14. Dalton HJ, Garcia-Filion P, Holubkov R, Moler FW, Shanley T, Heidemann S, et al. Association of bleeding and thrombosis with outcome in extracorporeal life support. Pediatr Crit Care Med. 2015; 16:167–174.
Article15. Ranucci M. Coagulation, anticoagulation and inflammatory response. 1st ed. Milan: Springer;2014.16. Bembea MM, Schwartz JM, Shah N, Colantuoni E, Lehmann CU, Kickler T, et al. Anticoagulation monitoring during pediatric extracorporeal membrane oxygenation. ASAIO J. 2013; 59:63–68.
Article17. Irby K, Swearingen C, Byrnes J, Bryant J, Prodhan P, Fiser R. Unfractionated heparin activity measured by anti-factor Xa levels is associated with the need for extracorporeal membrane oxygenation circuit/membrane oxygenator change: a retrospective pediatric study. Pediatr Crit Care Med. 2014; 15:e175–e182.18. Kim DW, Cheon KR, Cho D, Lee KS, Cho HJ, Jeong IS. Transfusion associated hyperkalemia and cardiac arrest in an infant after extracorporeal membrane oxygenation. Korean J Crit Care Med. 2015; 30:132–134.
Article19. Ryerson LM, Lequier LL. Anticoagulation management and monitoring during pediatric extracorporeal life support: a review of current issues. Front Pediatr. 2016; 4:67.
Article20. Cho HJ, Kim BY, Song ES, Oh SG, Oh BS, Jeong IS. Fatal left ventricular thrombosis in an infant receiving extracorporeal membrane oxygenation support: a case report. Korean J Crit Care Med. 2013; 28:123–126.
Article21. Pratt CW, Church FC. Antithrombin: structure and function. Semin Hematol. 1991; 28:3–9.22. Boisclair MD, Lane DA, Philippou H, Esnouf MP, Sheikh S, Hunt B, et al. Mechanisms of thrombin generation during surgery and cardiopulmonary bypass. Blood. 1993; 82:3350–3357.
Article23. Edmunds LH Jr, Colman RW. Thrombin during cardiopulmonary bypass. Ann Thorac Surg. 2006; 82:2315–2322.
Article24. Stansfield BK, Wise L, Ham PB 3rd, Patel P, Parman M, Jin C, et al. Outcomes following routine antithrombin III replacement during neonatal extracorporeal membrane oxygenation. J Pediatr Surg. 2017; 52:609–613.
Article25. Bates SM, Weitz JI. The mechanism of action of thrombin inhibitors. J Invasive Cardiol. 2000; 12:Suppl F. 27F–32F.26. Murdoch IA, Beattie RM, Silver DM. Heparin-induced thrombocytopenia in children. Acta Paediatr. 1993; 82:495–497.
Article27. Potter C, Gill JC, Scott JP, McFarland JG. Heparin-induced thrombocytopenia in a child. J Pediatr. 1992; 121:135–138.
Article28. Levi M, Bijsterveld NR, Keller TT. Recombinant factor VIIa as an antidote for anticoagulant treatment. Semin Hematol. 2004; 41:1 Suppl 1. 65–69.
Article29. Young G, Yonekawa KE, Nakagawa PA, Blain RC, Lovejoy AE, Nugent DJ. Recombinant activated factor VII effectively reverses the anticoagulant effects of heparin, enoxaparin, fondaparinux, argatroban, and bivalirudin ex vivo as measured using thromboelastography. Blood Coagul Fibrinolysis. 2007; 18:547–553.
Article30. Shantsila E, Lip GY, Chong BH. Heparin-induced thrombocytopenia. A contemporary clinical approach to diagnosis and management. Chest. 2009; 135:1651–1664.31. Sanfilippo F, Asmussen S, Maybauer DM, Santonocito C, Fraser JF, Erdoes G, et al. Bivalirudin for alternative anticoagulation in extracorporeal membrane oxygenation: a systematic review. J Intensive Care Med. 2017; 32:312–319.
Article32. Mirdamadi A. Dabigatran, a direct thrombin inhibitor, can be a life-saving treatment in heparin-induced thrombocytopenia. ARYA Atheroscler. 2013; 9:112–114.33. Nagle EL, Dager WE, Duby JJ, Roberts AJ, Kenny LE, Murthy MS, et al. Bivalirudin in pediatric patients maintained on extracorporeal life support. Pediatr Crit Care Med. 2013; 14:e182–e188.
Article34. Almond CS, Harrington J, Thiagarajan R, Duncan CN, LaPierre R, Halwick D, et al. Successful use of bivalirudin for cardiac transplantation in a child with heparin-induced thrombocytopenia. J Heart Lung Transplant. 2006; 25:1376–1379.
Article35. Young G, Tarantino MD, Wohrley J, Weber LC, Belvedere M, Nugent DJ. Pilot dose-finding and safety study of bivalirudin in infants <6 months of age with thrombosis. J Thromb Haemost. 2007; 5:1654–1659.
Article36. Pieri M, Agracheva N, Bonaveglio E, Greco T, De Bonis M, Covello RD, et al. Bivalirudin versus heparin as an anticoagulant during extracorporeal membrane oxygenation: a case-control study. J Cardiothorac Vasc Anesth. 2013; 27:30–34.
Article37. Ranucci M, Ballotta A, Kandil H, Isgro G, Carlucci C, Baryshnikova E, et al. Bivalirudin-based versus conventional heparin anticoagulation for postcardiotomy extracorporeal membrane oxygenation. Crit Care. 2011; 15:R275.
Article38. Lewis BE, Hursting MJ. Argatroban therapy in heparin-induced thrombocytopenia. In : Greinacher A, Warkentin TE, editors. Heparin-induced Thrombocytopenia. 3rd ed. New York: Marcel Dekker;2004.39. Madabushi R, Cox DS, Hossain M, Boyle DA, Patel BR, Young G, et al. Pharmacokinetic and pharmacodynamic basis for effective argatroban dosing in pediatrics. J Clin Pharmacol. 2011; 51:19–28.
Article40. Swan SK, Hursting MJ. The pharmacokinetics and pharmacodynamics of argatroban: effects of age, gender, and hepatic or renal dysfunction. Pharmacotherapy. 2000; 20:318–329.
Article41. Kawada T, Kitagawa H, Hoson M, Okada Y, Shiomura J. Clinical application of argatroban as an alternative anticoagulant for extracorporeal circulation. Hematol Oncol Clin North Am. 2000; 14:445–457. x
Article42. Hursting MJ, Dubb J, Verme-Gibboney CN. Argatroban anticoagulation in pediatric patients: a literature analysis. J Pediatr Hematol Oncol. 2006; 28:4–10.43. Kitagawa H, Chang H, Fujita T. Hyperkalemia due to nafamostat mesylate. N Engl J Med. 1995; 332:687.
Article44. Muto S, Imai M, Asano Y. Mechanisms of hyperkalemia caused by nafamostat mesilate. Gen Pharmacol. 1995; 26:1627–1632.
Article45. Lim JY, Kim JB, Choo SJ, Chung CH, Lee JW, Jung SH. Anticoagulation during extracorporeal membrane oxygenation; nafamostat mesilate versus heparin. Ann Thorac Surg. 2016; 102:534–539.
Article46. Park JH, Her C, Min HK, Kim DK, Park SH, Jang HJ. Nafamostat mesilate as a regional anticoagulant in patients with bleeding complications during extracorporeal membrane oxygenation. Int J Artif Organs. 2015; 38:595–599.
Article47. Han SJ, Kim HS, Kim KI, Whang SM, Hong KS, Lee WK, et al. Use of nafamostat mesilate as an anticoagulant during extracorporeal membrane oxygenation. J Korean Med Sci. 2011; 26:945–950.
Article48. Nagaya M, Futamura M, Kato J, Niimi N, Fukuta S. Application of a new anticoagulant (Nafamostat Mesilate) to control hemorrhagic complications during extracorporeal membrane oxygenation--a preliminary report. J Pediatr Surg. 1997; 32:531–535.
Article49. Larsson M, Rayzman V, Nolte MW, Nickel KF, Bjorkqvist J, Jamsa A, et al. A factor XIIa inhibitory antibody provides thromboprotection in extracorporeal circulation without increasing bleeding risk. Sci Transl Med. 2014; 6:222ra17.
Article50. Baird CW, Zurakowski D, Robinson B, Gandhi S, Burdis-Koch L, Tamblyn J, et al. Anticoagulation and pediatric extracorporeal membrane oxygenation: impact of activated clotting time and heparin dose on survival. Ann Thorac Surg. 2007; 83:912–919. discussion 919-20.
Article51. Kuhle S, Eulmesekian P, Kavanagh B, Massicotte P, Vegh P, Lau A, et al. Lack of correlation between heparin dose and standard clinical monitoring tests in treatment with unfractionated heparin in critically ill children. Haematologica. 2007; 92:554–557.
Article52. Kim GG, El Rouby S, Thompson J, Gupta A, Williams J, Jobes DR. Monitoring unfractionated heparin in pediatric patients with congenital heart disease having cardiac catheterization or cardiac surgery. J Thromb Thrombolysis. 2010; 29:429–436.
Article53. Maul TM, Wolff EL, Kuch BA, Rosendorff A, Morell VO, Wearden PD. Activated partial thromboplastin time is a better trending tool in pediatric extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2012; 13:e363–e371.
Article54. Liveris A, Bello RA, Friedmann P, Duffy MA, Manwani D, Killinger JS, et al. Anti-factor Xa assay is a superior correlate of heparin dose than activated partial thromboplastin time or activated clotting time in pediatric extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2014; 15:e72–e79.
Article55. Northrop MS, Sidonio RF, Phillips SE, Smith AH, Daphne HC, Pietsch JB, et al. The use of an extracorporeal membrane oxygenation anticoagulation laboratory protocol is associated with decreased blood product use, decreased hemorrhagic complications, and increased circuit life. Pediatr Crit Care Med. 2015; 16:66–74.
Article56. O'Meara LC, Alten JA, Goldberg KG, Timpa JG, Phillips J, Laney D, et al. Anti-xa directed protocol for anticoagulation management in children supported with extracorporeal membrane oxygenation. ASAIO J. 2015; 61:339–344.57. Kostousov V, Nguyen K, Hundalani SG, Teruya J. The influence of free hemoglobin and bilirubin on heparin monitoring by activated partial thromboplastin time and anti-Xa assay. Arch Pathol Lab Med. 2014; 138:1503–1506.
Article58. Newall F, Ignjatovic V, Johnston L, Summerhayes R, Lane G, Cranswick N, et al. Clinical use of unfractionated heparin therapy in children: time for change? Br J Haematol. 2010; 150:674–678.
Article59. Urlesberger B, Zobel G, Zenz W, Kuttnig-Haim M, Maurer U, Reiterer F, et al. Activation of the clotting system during extracorporeal membrane oxygenation in term newborn infants. J Pediatr. 1996; 129:264–268.
Article60. Muntean W. Coagulation and anticoagulation in extracorporeal membrane oxygenation. Artif Organs. 1999; 23:979–983.
Article61. Ganter MT, Hofer CK. Coagulation monitoring: current techniques and clinical use of viscoelastic point-of-care coagulation devices. Anesth Analg. 2008; 106:1366–1375.
Article62. Saini A, Hartman ME, Gage BF, Said A, Gazit AZ, Eghtesady P, et al. Incidence of platelet dysfunction by thromboelastography-platelet mapping in children supported with ECMO: a pilot retrospective study. Front Pediatr. 2016; 3:116.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Change of Platelet Count and Plasma Fibrinogen Level during and after Extracorporeal Circulation
- Experiences of Tracheal Procedure Assisted by Extracorporeal Membrane Oxygenator
- Successful Resuscitation of Prolonged Cardiac Arrest Using Emergency Extracorporeal Membrane Oxygenator: A case report
- The Effect of Venoarterial Extracorporeal Lung Assist ( ECLA ) Using a Small - sized Membrane Oxygenator in Hypoxic Dogs
- Prolonged Extracorporeal Lung Heart Assist ( Extracorporeal Membrane Oxygenation ) - 4 cases report